A central role for RAF→MEK→ERK signaling in the genesis of pancreatic ductal adenocarcinoma
- PMID: 22628411
- PMCID: PMC3425446
- DOI: 10.1158/2159-8290.CD-11-0347
A central role for RAF→MEK→ERK signaling in the genesis of pancreatic ductal adenocarcinoma
Abstract
KRAS mutation is a hallmark of pancreatic ductal adenocarcinoma (PDA) but remains an intractable pharmacologic target. Consequently, defining RAS effector pathway(s) required for PDA initiation and maintenance is critical to improve treatment of this disease. Here, we show that expression of BRAF(V600E), but not PIK3CA(H1047R), in the mouse pancreas leads to pancreatic intraepithelial neoplasia (PanIN) lesions. Moreover, concomitant expression of BRAF(V600E) and TP53(R270H) result in lethal PDA. We tested pharmacologic inhibitors of RAS effectors against multiple human PDA cell lines. Mitogen-activated protein (MAP)/extracellular signal-regulated (ERK) kinase (MEK) inhibition was highly effective both in vivo and in vitro and was synergistic with AKT inhibition in most cell lines tested. We show that RAF→MEK→ERK signaling is central to the initiation and maintenance of PDA and to rational combination strategies in this disease. These results emphasize the value of leveraging multiple complementary experimental systems to prioritize pathways for effective intervention strategies in PDA.
Significance: PDA is diffi cult to treat, in large part, due to recurrent mutations in the KRAS gene. Here, we defi ne rational treatment approaches for the disease achievable today with existing drug combinations by thorough genetic and pharmacologic dissection of the major KRAS effector pathways, RAF→MEK→ERK and phosphoinositide 3′-kinase (PI3'K)→AKT.
Figures
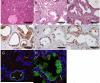

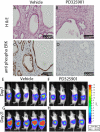
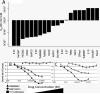
Comment in
-
RAF/MEK dependence of KRAS-mutant pancreatic ductal adenocarcinomas.Cancer Discov. 2012 Aug;2(8):666-9. doi: 10.1158/2159-8290.CD-12-0308. Cancer Discov. 2012. PMID: 22886659 Free PMC article.
References
-
- Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes & development. 2006;20:1218–49. - PubMed
-
- Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–54. - PubMed
-
- Yanagisawa A, Ohtake K, Ohashi K, Hori M, Kitagawa T, Sugano H, et al. Frequent c-Ki-ras oncogene activation in mucous cell hyperplasias of pancreas suffering from chronic inflammation. Cancer Res. 1993;53:953–6. - PubMed
-
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–50. - PubMed
-
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

