Activating transcription factor 4 regulates stearate-induced vascular calcification
- PMID: 22628618
- PMCID: PMC3540843
- DOI: 10.1194/jlr.M025981
Activating transcription factor 4 regulates stearate-induced vascular calcification
Abstract
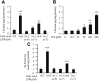
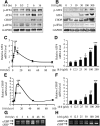
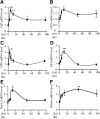
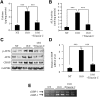
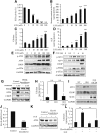
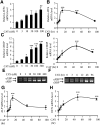
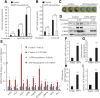
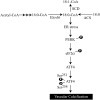
Previously, we reported that stearate, a saturated fatty acid, promotes osteoblastic differentiation and mineralization of vascular smooth muscle cells (VSMC). In this study, we examined the molecular mechanisms by which stearate promotes vascular calcification. ATF4 is a pivotal transcription factor in osteoblastogenesis and endoplasmic reticulum (ER) stress. Increased stearate by either supplementation of exogenous stearic acid or inhibition of stearoyl-CoA desaturase (SCD) by CAY10566 induced ATF4 mRNA, phosphorylated ATF4 protein, and total ATF4 protein. Induction occurred through activation of the PERK-eIF2α pathway, along with increased osteoblastic differentiation and mineralization of VSMCs. Either stearate or the SCD inhibitor but not oleate or other fatty acid treatments also increased ER stress as determined by the expression of p-eIF2α, CHOP, and the spliced form of XBP-1, which were directly correlated with ER stearate levels. ATF4 knockdown by lentiviral ATF4 shRNA blocked osteoblastic differentiation and mineralization induced by stearate and SCD inhibition. Conversely, treatment of VSMCs with an adenovirus containing ATF4 induced vascular calcification. Our results demonstrated that activation of ATF4 mediates vascular calcification induced by stearate.
Figures

References
-
- Mizobuchi M., Towler D., Slatopolsky E. 2009. Vascular calcification: the killer of patients with chronic kidney disease. J. Am. Soc. Nephrol. 20: 1453–1464 - PubMed
-
- Li X., Yang H. Y., Giachelli C. M. 2006. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ. Res. 98: 905–912 - PubMed
-
- Tintut Y., Patel J., Parhami F., Demer L. L. 2000. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 102: 2636–2642 - PubMed
-
- Luo G., Ducy P., McKee M. D., Pinero G. J., Loyer E., Behringer R. R., Karsenty G. 1997. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 386: 78–81 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

