Biochemical aspects of a serine protease from Caesalpinia echinata Lam. (Brazilwood) seeds: a potential tool to access the mobilization of seed storage proteins
- PMID: 22629147
- PMCID: PMC3354420
- DOI: 10.1100/2012/562715
Biochemical aspects of a serine protease from Caesalpinia echinata Lam. (Brazilwood) seeds: a potential tool to access the mobilization of seed storage proteins
Abstract
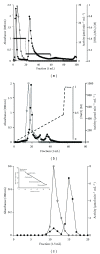
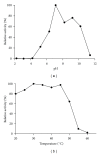
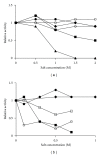
Several proteins have been isolated from seeds of leguminous, but this is the first report that a protease was obtained from seeds of Caesalpinia echinata Lam., a tree belonging to the Fabaceae family. This enzyme was purified to homogeneity by hydrophobic interaction and anion exchange chromatographies and gel filtration. This 61-kDa serine protease (CeSP) hydrolyses H-D-prolyl-L-phenylalanyl-L-arginine-p-nitroanilide (K(m) 55.7 μM) in an optimum pH of 7.1, and this activity is effectively retained until 50 °C. CeSP remained stable in the presence of kosmotropic anions (PO(4) (3-), SO(4) (2-), and CH(3)COO(-)) or chaotropic cations (K(+) and Na(+)). It is strongly inhibited by TLCK, a serine protease inhibitor, but not by E-64, EDTA or pepstatin A. The characteristics of the purified enzyme allowed us to classify it as a serine protease. The role of CeSP in the seeds cannot be assigned yet but is possible to infer that it is involved in the mobilization of seed storage proteins.
Figures

References
-
- Schaller A. A cut above the rest: the regulatory function of plant proteases. Planta. 2004;220(2):183–197. - PubMed
-
- Nishikata M. Trypsin-like protease from soybean seeds. purification and some properties. Journal of Biochemistry. 1984;95(4):1169–1177. - PubMed
-
- Oshikawa K, Aoki K-I, Yoshino Y, Terada S. Purification and characterization of a basic amino acid-specific peptidase from seeds of jack bean (Canavalia ensiformis) Bioscience, Biotechnology and Biochemistry. 2000;64(10):2186–2192. - PubMed
-
- Demartini DR, Wlodawer A, Carlini CR. A comparative study of the expression of serine proteinases in quiescent seeds and in developing Canavalia ensiformis plants. Journal of Experimental Botany. 2007;58(3):521–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

