Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork
- PMID: 22629359
- PMCID: PMC3357388
- DOI: 10.1371/journal.pone.0037150
Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork
Abstract
Shiftwork is associated with adverse metabolic pathophysiology, and the rising incidence of shiftwork in modern societies is thought to contribute to the worldwide increase in obesity and metabolic syndrome. The underlying mechanisms are largely unknown, but may involve direct physiological effects of nocturnal light exposure, or indirect consequences of perturbed endogenous circadian clocks. This study employs a two-week paradigm in mice to model the early molecular and physiological effects of shiftwork. Two weeks of timed sleep restriction has moderate effects on diurnal activity patterns, feeding behavior, and clock gene regulation in the circadian pacemaker of the suprachiasmatic nucleus. In contrast, microarray analyses reveal global disruption of diurnal liver transcriptome rhythms, enriched for pathways involved in glucose and lipid metabolism and correlating with first indications of altered metabolism. Although altered food timing itself is not sufficient to provoke these effects, stabilizing peripheral clocks by timed food access can restore molecular rhythms and metabolic function under sleep restriction conditions. This study suggests that peripheral circadian desynchrony marks an early event in the metabolic disruption associated with chronic shiftwork. Thus, strengthening the peripheral circadian system by minimizing food intake during night shifts may counteract the adverse physiological consequences frequently observed in human shift workers.
Conflict of interest statement
Figures
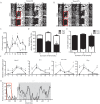


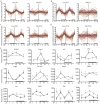
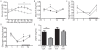
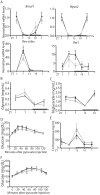
References
-
- Baron KG, Reid KJ, Kern AS, Zee PC. Role of Sleep Timing in Caloric Intake and BMI. Obesity. 2011;19:1374–1381. - PubMed
-
- Li Y, Sato Y, Yamaguchi N. Shift work and the risk of metabolic syndrome: a nested case-control study. Int J Occup Environ Health. 2011;17:154–160. - PubMed
-
- Garaulet M, Ortega FB, Ruiz JR, Rey-Lopez JP, Beghin L, et al. The HELENA study. Int J Obes; 2011. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

