Induction of selective blood-tumor barrier permeability and macromolecular transport by a biostable kinin B1 receptor agonist in a glioma rat model
- PMID: 22629405
- PMCID: PMC3357387
- DOI: 10.1371/journal.pone.0037485
Induction of selective blood-tumor barrier permeability and macromolecular transport by a biostable kinin B1 receptor agonist in a glioma rat model
Erratum in
- PLoS One. 2012;7(6): doi/10.1371/annotation/6b95427c-645d-4f1b-a648-ceb215129583
Abstract
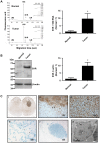
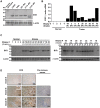
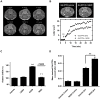
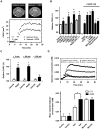
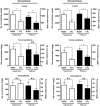
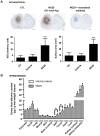
Treatment of malignant glioma with chemotherapy is limited mostly because of delivery impediment related to the blood-brain tumor barrier (BTB). B1 receptors (B1R), inducible prototypical G-protein coupled receptors (GPCR) can regulate permeability of vessels including possibly that of brain tumors. Here, we determine the extent of BTB permeability induced by the natural and synthetic peptide B1R agonists, LysdesArg(9)BK (LDBK) and SarLys[dPhe(8)]desArg(9)BK (NG29), in syngeneic F98 glioma-implanted Fischer rats. Ten days after tumor inoculation, we detected the presence of B1R on tumor cells and associated vasculature. NG29 infusion increased brain distribution volume and uptake profiles of paramagnetic probes (Magnevist and Gadomer) at tumoral sites (T(1)-weighted imaging). These effects were blocked by B1R antagonist and non-selective cyclooxygenase inhibitors, but not by B2R antagonist and non-selective nitric oxide synthase inhibitors. Consistent with MRI data, systemic co-administration of NG29 improved brain tumor delivery of Carboplatin chemotherapy (ICP-Mass spectrometry). We also detected elevated B1R expression in clinical samples of high-grade glioma. Our results documented a novel GPCR-signaling mechanism for promoting transient BTB disruption, involving activation of B1R and ensuing production of COX metabolites. They also underlined the potential value of synthetic biostable B1R agonists as selective BTB modulators for local delivery of different sized-therapeutics at (peri)tumoral sites.
Conflict of interest statement
Figures
References
-
- Maroldi R, Ambrosi C, Farina D. Metastatic disease of the brain: extra-axial metastases (skull, dura, leptomeningeal) and tumour spread. Eur Radiol. 2005;15:617–626. - PubMed
-
- Rainov NG, Soling A, Heidecke V. Novel therapies for malignant gliomas: a local affair? Neurosurg Focus. 2006;20:E9. - PubMed
-
- Rich JN, Bigner DD. Development of novel targeted therapies in the treatment of malignant glioma. Nat Rev Drug Discov. 2004;3:430–446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

