Paxillin and Hic-5 interaction with vinculin is differentially regulated by Rac1 and RhoA
- PMID: 22629471
- PMCID: PMC3358283
- DOI: 10.1371/journal.pone.0037990
Paxillin and Hic-5 interaction with vinculin is differentially regulated by Rac1 and RhoA
Abstract
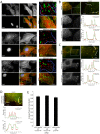
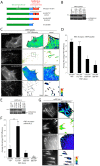
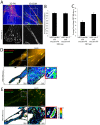
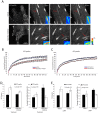
Cell migration is of paramount importance to organism development and maintenance as well as multiple pathological processes, including cancer metastasis. The RhoGTPases Rac1 and RhoA are indispensable for cell migration as they regulate cell protrusion, cell-extracellular matrix (ECM) interactions and force transduction. However, the consequences of their activity at a molecular level within the cell remain undetermined. Using a combination of FRET, FRAP and biochemical analyses we show that the interactions between the focal adhesion proteins vinculin and paxillin, as well as the closely related family member Hic-5 are spatially and reciprocally regulated by the activity of Rac1 and RhoA. Vinculin in its active conformation interacts with either paxillin or Hic-5 in adhesions in response to Rac1 and RhoA activation respectively, while inactive vinculin interacts with paxillin in the membrane following Rac1 inhibition. Additionally, Rac1 specifically regulates the dynamics of paxillin as well as its binding partner and F-actin interacting protein actopaxin (α-parvin) in adhesions. Furthermore, FRET analysis of protein:protein interactions within cell adhesions formed in 3D matrices revealed that, in contrast to 2D systems vinculin interacts preferentially with Hic-5. This study provides new insight into the complexity of cell-ECM adhesions in both 2D and 3D matrices by providing the first description of RhoGTPase-coordinated protein:protein interactions in a cellular microenvironment. These data identify discrete roles for paxillin and Hic-5 in Rac1 and RhoA-dependent cell adhesion formation and maturation; processes essential for productive cell migration.
Conflict of interest statement
Figures




References
-
- Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. - PubMed
-
- Kim C, Ye F, Ginsberg MH. Regulation of integrin activation. Annu Rev Cell Dev Biol. 2011;27:321–345. - PubMed
-
- Brown MC, Turner CE. Paxillin: Adapting to change. Physiol Rev. 2004;84(4):1315–1339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

