Emerging drugs for Duchenne muscular dystrophy
- PMID: 22632414
- PMCID: PMC3486431
- DOI: 10.1517/14728214.2012.691965
Emerging drugs for Duchenne muscular dystrophy
Abstract
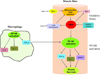
Introduction: Duchenne muscular dystrophy (DMD) is the most common, severe childhood form of muscular dystrophy. Treatment is limited to glucocorticoids that have the benefit of prolonging ambulation by approximately 2 years and preventing scoliosis. Finding a more satisfactory treatment should focus on maintaining long-term efficacy with a minimal side effect profile.
Areas covered: Authors discuss different therapeutic strategies that have been used in pre-clinical and clinical settings.
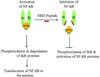
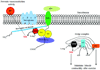
Expert opinion: Multiple treatment approaches have emerged. Most attractive are molecular-based therapies that can express the missing dystrophin protein (exon skipping or mutation suppression) or a surrogate gene product (utrophin). Other approaches include increasing the strength of muscles (myostatin inhibitors), reducing muscle fibrosis and decreasing oxidative stress. Additional targets include inhibiting NF-κB to reduce inflammation or promoting skeletal muscle blood flow and muscle contractility using phosphodiesterase inhibitors or nitric oxide (NO) donors. The potential for each of these treatment strategies to enter clinical trials is a central theme of discussion. The review emphasizes that the goal of treatment should be to find a product at least as good as glucocorticoids with a lower side effect profile or with a significant glucocorticoid sparing effect.
Conflict of interest statement
All other authors state no conflict of interest and have received no payment in preparation of this manuscript.
Figures
References
-
- Prevalence of Duchenne/Becker muscular dystrophy among males aged 5–24 years - four states, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:1119–1122. - PubMed
-
-
Mendell JR, Shilling C, Leslie ND, et al. Evidence-based path to newborn screening for duchenne muscular dystrophy. Ann Neurol. 2012;71:304–313. First report of two-tier system using dried blood spots taken at birth to identify DMD cases. CK is first determined and if it meets a threshold preditive of DMD gene mutations, DNA testing will follow on the same dried blood spot sample.
-
-
- Brooke MH, Fenichel GM, Griggs RC, et al. Clinical investigation in Duchenne dystrophy: 2. Determination of the "power" of therapeutic trials based on the natural history. Muscle Nerve. 1983;6:91–103. - PubMed
-
- Eagle M, Baudouin SV, Chandler C, et al. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12:926–929. - PubMed
-
-
England SB, Nicholson LV, Johnson MA, et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;343:180–182. Case report of interest demonstrating a mild case of Becker muscular dystrophy with almost one-half of the DMD gene deleted.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
