Loss of plakoglobin promotes decreased cell-cell contact, increased invasion, and breast cancer cell dissemination in vivo
- PMID: 22632416
- PMCID: PMC3446349
- DOI: 10.1186/bcr3201
Loss of plakoglobin promotes decreased cell-cell contact, increased invasion, and breast cancer cell dissemination in vivo
Erratum in
-
Erratum to: Loss of plakoglobin promotes cell-cell contact, increased invasion and breast cancer cell dissemination in vivo.Breast Cancer Res. 2017 Mar 28;19(1):37. doi: 10.1186/s13058-017-0835-4. Breast Cancer Res. 2017. PMID: 28351370 Free PMC article. No abstract available.
Abstract
Introduction: The majority of deaths from breast cancer are a result of metastases; however, little is understood about the genetic alterations underlying their onset. Genetic profiling has identified the adhesion molecule plakoglobin as being three-fold reduced in expression in primary breast tumors that have metastasized compared with nonmetastatic tumors. In this study, we demonstrate a functional role for plakoglobin in the shedding of tumor cells from the primary site into the circulation.
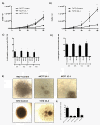
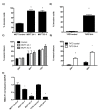
Methods: We investigated the effects of plakoglobin knockdown on breast cancer cell proliferation, migration, adhesion, and invasion in vitro and on tumor growth and intravasation in vivo. MCF7 and T47D cells were stably transfected with miRNA sequences targeting the plakoglobin gene, or scramble vector. Gene and protein expression was monitored by quantitative polymerase chain reaction (qPCR) and Western blot. Cell proliferation, adhesion, migration, and invasion were measured by cell counting, flow cytometry, and scratch and Boyden Chamber assays. For in vivo experiments, plakoglobin knockdown and control cells were inoculated into mammary fat pads of mice, and tumor growth, shedding of tumor cells into the bloodstream, and evidence of metastatic bone lesions were monitored with caliper measurement, flow cytometry, and microcomputed tomography (μCT), respectively.
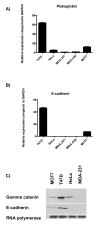
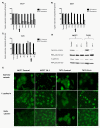
Results: Plakoglobin and γ-catenin expression were reduced by more than 80% in all knockdown cell lines used but were unaltered after transfection with the scrambled sequence. Reduced plakoglobin resulted in significantly increased in MCF7 and T47D cell proliferation in vitro and in vivo, compared with control, with significantly more tumor cells being shed into the bloodstream of mice bearing plakoglobin knockdown tumors. In addition, plakoglobin knockdown cells showed a >250% increase in invasion through basement membrane and exhibited reduced cell-to-cell adhesion compared with control cells.
Conclusion: Decreased plakoglobin expression increases the invasive behavior of breast cancer cells. This is the first demonstration of a functional role for plakoglobin/γ-catenin in the metastatic process, indicating that this molecule may represent a target for antimetastatic therapies.
Figures

Similar articles
-
KRT13 promotes stemness and drives metastasis in breast cancer through a plakoglobin/c-Myc signaling pathway.Breast Cancer Res. 2022 Jan 25;24(1):7. doi: 10.1186/s13058-022-01502-6. Breast Cancer Res. 2022. PMID: 35078507 Free PMC article.
-
Beyond cell-cell adhesion: Plakoglobin and the regulation of tumorigenesis and metastasis.Oncotarget. 2017 May 9;8(19):32270-32291. doi: 10.18632/oncotarget.15650. Oncotarget. 2017. PMID: 28416759 Free PMC article. Review.
-
JMJD2A contributes to breast cancer progression through transcriptional repression of the tumor suppressor ARHI.Breast Cancer Res. 2014 May 30;16(3):R56. doi: 10.1186/bcr3667. Breast Cancer Res. 2014. PMID: 24886710 Free PMC article.
-
Histone deacetylase 7 inhibits plakoglobin expression to promote lung cancer cell growth and metastasis.Int J Oncol. 2019 Mar;54(3):1112-1122. doi: 10.3892/ijo.2019.4682. Epub 2019 Jan 9. Int J Oncol. 2019. PMID: 30628670
-
Circulating tumor cell clusters-associated gene plakoglobin and breast cancer survival.Breast Cancer Res Treat. 2015 Jun;151(3):491-500. doi: 10.1007/s10549-015-3416-1. Epub 2015 May 10. Breast Cancer Res Treat. 2015. PMID: 25957595 Review.
Cited by
-
KRT13 promotes stemness and drives metastasis in breast cancer through a plakoglobin/c-Myc signaling pathway.Breast Cancer Res. 2022 Jan 25;24(1):7. doi: 10.1186/s13058-022-01502-6. Breast Cancer Res. 2022. PMID: 35078507 Free PMC article.
-
Modulation of cytoskeletal dynamics by mammalian nucleoside diphosphate kinase (NDPK) proteins.Naunyn Schmiedebergs Arch Pharmacol. 2015 Feb;388(2):189-97. doi: 10.1007/s00210-014-1046-5. Epub 2014 Sep 20. Naunyn Schmiedebergs Arch Pharmacol. 2015. PMID: 25234227 Free PMC article. Review.
-
Beyond cell-cell adhesion: Plakoglobin and the regulation of tumorigenesis and metastasis.Oncotarget. 2017 May 9;8(19):32270-32291. doi: 10.18632/oncotarget.15650. Oncotarget. 2017. PMID: 28416759 Free PMC article. Review.
-
Alternative splicing regulation and its therapeutic potential in bladder cancer.Front Oncol. 2024 Jul 26;14:1402350. doi: 10.3389/fonc.2024.1402350. eCollection 2024. Front Oncol. 2024. PMID: 39132499 Free PMC article. Review.
-
The elevated transcription of ADAM19 by the oncohistone H2BE76K contributes to oncogenic properties in breast cancer.J Biol Chem. 2021 Jan-Jun;296:100374. doi: 10.1016/j.jbc.2021.100374. Epub 2021 Feb 4. J Biol Chem. 2021. PMID: 33548228 Free PMC article.
References
-
- Woelfle U, Cloos J, Sauter G, Riethdorf L, Jänicke F, van Diest P, Brakenhoff R, Pantel K. Molecular signature associated with bone marrow micrometastasis in human breast cancer. Cancer Res. 2003;63:5679–5684. - PubMed
-
- Aberle H, Bierkamp C, Torchard D, Serova O, Wagner T, Natt E, Wirsching J, Heidkämper C, Montagna M, Lynch HT. The human plakoglobin gene localizes on chromosome 17q21 and is subjected to loss of heterozygosity in breast and ovarian cancers. Proc Natl Acad Sci USA. 1995;92:6384–6388. doi: 10.1073/pnas.92.14.6384. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

