Fluorescence-guided surgery allows for more complete resection of pancreatic cancer, resulting in longer disease-free survival compared with standard surgery in orthotopic mouse models
- PMID: 22632917
- PMCID: PMC3383387
- DOI: 10.1016/j.jamcollsurg.2012.02.021
Fluorescence-guided surgery allows for more complete resection of pancreatic cancer, resulting in longer disease-free survival compared with standard surgery in orthotopic mouse models
Abstract
Background: Negative surgical margins are vital to achieve cure and prolong survival in patients with pancreatic cancer. We inquired if fluorescence-guided surgery (FGS) could improve surgical outcomes and reduce recurrence rates in orthotopic mouse models of human pancreatic cancer.
Study design: A randomized active-control preclinical trial comparing bright light surgery (BLS) to FGS was used. Orthotopic mouse models of human pancreatic cancer were established using the BxPC-3 pancreatic cancer cell line expressing red fluorescent protein (RFP). Two weeks after orthotopic implantation, tumors were resected with BLS or FGS. Pre- and postoperative images were obtained with the OV-100 Small Animal Imaging System to assess completeness of surgical resection in real time. Postoperatively, noninvasive whole body imaging was done to assess recurrence and follow tumor progression. Six weeks postoperatively, mice were sacrificed to evaluate primary pancreatic and metastatic tumor burden at autopsy.
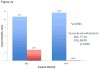
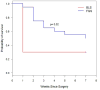
Results: A more complete resection of pancreatic cancer was achieved using FGS compared with BLS: 98.9% vs 77.1%, p = 0.005. The majority of mice undergoing BLS (63.2%) had evidence of gross disease with no complete resections; 20% of mice undergoing FGS had complete resection and an additional 75% had only minimal residual disease (p = 0.0001). The mean postoperative tumor burden was significantly less with FGS compared with BLS: 0.08 ± 0.06 mm(2) vs 2.64 ± 0.63 mm(2), p = 0.001. The primary tumor burden at termination was significantly less with FGS compared with BLS: 19.3 ± 5.3 mm(2) vs 6.2 ± 3.6 mm(2), p = 0.048. FGS resulted in significantly longer disease-free survival than BLS (p = 0.02, hazard ratio = 0.39, 95% CI 0.17, 0.88).
Conclusions: Surgical outcomes were improved in pancreatic cancer using fluorescence-guidance. This novel approach has significant potential to improve surgical treatment of cancer.
Copyright © 2012 American College of Surgeons. Published by Elsevier Inc. All rights reserved.
Figures





Comment in
-
Reality or dream of fluorescence-guided pancreatic cancer surgery?J Am Coll Surg. 2012 Oct;215(4):591; author reply 592-3. doi: 10.1016/j.jamcollsurg.2012.07.001. J Am Coll Surg. 2012. PMID: 22981066 No abstract available.
Similar articles
-
Fluorescence-guided surgery with a fluorophore-conjugated antibody to carcinoembryonic antigen (CEA), that highlights the tumor, improves surgical resection and increases survival in orthotopic mouse models of human pancreatic cancer.Ann Surg Oncol. 2014 Apr;21(4):1405-11. doi: 10.1245/s10434-014-3495-y. Epub 2014 Feb 6. Ann Surg Oncol. 2014. PMID: 24499827 Free PMC article.
-
Fluorescence-guided surgery of human colon cancer increases complete resection resulting in cures in an orthotopic nude mouse model.J Surg Res. 2013 Jan;179(1):87-93. doi: 10.1016/j.jss.2012.08.052. Epub 2012 Sep 7. J Surg Res. 2013. PMID: 23079571 Free PMC article.
-
Fluorescence-guided surgery in combination with UVC irradiation cures metastatic human pancreatic cancer in orthotopic mouse models.PLoS One. 2014 Jun 12;9(6):e99977. doi: 10.1371/journal.pone.0099977. eCollection 2014. PLoS One. 2014. PMID: 24924955 Free PMC article.
-
Toward Curative Fluorescence-Guided Surgery of Pancreatic Cancer.Hepatogastroenterology. 2015 May;62(139):715-22. Hepatogastroenterology. 2015. PMID: 26897960 Review.
-
Should all distal pancreatectomies be performed laparoscopically?Adv Surg. 2009;43:283-300. doi: 10.1016/j.yasu.2009.02.013. Adv Surg. 2009. PMID: 19845186 Review.
Cited by
-
Shape memory polymers with visible and near-infrared imaging modalities: Synthesis, characterization and in vitro analysis.RSC Adv. 2017;7(32):19742-19753. doi: 10.1039/C6RA28165F. Epub 2017 Apr 4. RSC Adv. 2017. PMID: 30288254 Free PMC article.
-
Fluorescently labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model.J Surg Oncol. 2014 Apr;109(5):451-8. doi: 10.1002/jso.23507. Epub 2013 Nov 19. J Surg Oncol. 2014. PMID: 24249594 Free PMC article.
-
Fluorescent Anti-MUC5AC Brightly Targets Pancreatic Cancer in a Patient-derived Orthotopic Xenograft.In Vivo. 2022 Jan-Feb;36(1):57-62. doi: 10.21873/invivo.12676. In Vivo. 2022. PMID: 34972700 Free PMC article.
-
Peptide-Based Optical uPAR Imaging for Surgery: In Vivo Testing of ICG-Glu-Glu-AE105.PLoS One. 2016 Feb 1;11(2):e0147428. doi: 10.1371/journal.pone.0147428. eCollection 2016. PLoS One. 2016. PMID: 26828431 Free PMC article.
-
Fluorescence-guided surgery, but not bright-light surgery, prevents local recurrence in a pancreatic cancer patient derived orthotopic xenograft (PDOX) model resistant to neoadjuvant chemotherapy (NAC).Pancreatology. 2015 May-Jun;15(3):295-301. doi: 10.1016/j.pan.2015.02.008. Epub 2015 Mar 7. Pancreatology. 2015. PMID: 25800176 Free PMC article.
References
-
- Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. - PubMed
-
- Adham M, Jaeck D, Le Borgne J, et al. Long-term survival (5–20 years) after pancreatectomy for pancreatic ductal adenocarcinoma: a series of 30 patients collected from 3 institutions. Pancreas. 2008;37:352–357. - PubMed
-
- Cartwright T, Richards DA, Boehm KA. Cancer of the pancreas: are we making progress? A review of studies in the US Oncology Research Network. Cancer Control. 2008;15:308–313. - PubMed
-
- Di Marco M, Di Cicilia R, Macchini M, et al. Metastatic pancreatic cancer: is gemcitabine still the best standard treatment? (Review) Oncol Rep. 2010;23:1183–1192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

