Network crosstalk dynamically changes during neutrophil polarization
- PMID: 22632971
- PMCID: PMC3614011
- DOI: 10.1016/j.cell.2012.03.044
Network crosstalk dynamically changes during neutrophil polarization
Abstract
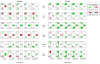
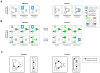
How complex signaling networks shape highly coordinated, multistep cellular responses is poorly understood. Here, we made use of a network-perturbation approach to investigate causal influences, or "crosstalk," among signaling modules involved in the cytoskeletal response of neutrophils to chemoattractant. We quantified the intensity and polarity of cytoskeletal marker proteins over time to characterize stereotyped cellular responses. Analyzing the effects of network disruptions revealed that, not only does crosstalk evolve rapidly during polarization, but also that intensity and polarity responses are influenced by different patterns of crosstalk. Interestingly, persistent crosstalk is arranged in a surprisingly simple circuit: a linear cascade from front to back to microtubules influences intensities, and a feed-forward network in the reverse direction influences polarity. Our approach provided a rational strategy for decomposing a complex, dynamically evolving signaling system and revealed evolving paths of causal influence that shape the neutrophil polarization response.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. - PubMed
-
- Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. - PubMed
-
- de Lichtenberg U, Jensen LJ, Brunak S, Bork P. Dynamic complex formation during the yeast cell cycle. Science. 2005;307:724–727. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

