A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly
- PMID: 22632975
- PMCID: PMC3361702
- DOI: 10.1016/j.cell.2012.03.039
A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly
Abstract
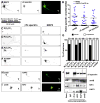
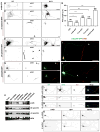
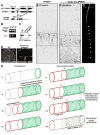
AnkyrinG (ankG) is highly enriched in neurons at axon initial segments (AISs) where it clusters Na(+) and K(+) channels and maintains neuronal polarity. How ankG becomes concentrated at the AIS is unknown. Here, we show that as neurons break symmetry, they assemble a distal axonal submembranous cytoskeleton, comprised of ankyrinB (ankB), αII-spectrin, and βII-spectrin, that defines a boundary limiting ankG to the proximal axon. Experimentally moving this boundary altered the length of ankG staining in the proximal axon, whereas disruption of the boundary through silencing of ankB, αII-spectrin, or βII-spectrin expression blocked AIS assembly and permitted ankG to redistribute throughout the distal axon. In support of an essential role for the distal cytoskeleton in ankG clustering, we also found that αII and βII-spectrin-deficient mice had disrupted AIS. Thus, the distal axonal cytoskeleton functions as an intra-axonal boundary restricting ankG to the AIS.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Neural development: No entry for AnkG.Nat Rev Neurosci. 2012 Jun 13;13(7):450. doi: 10.1038/nrn3282. Nat Rev Neurosci. 2012. PMID: 22691912 No abstract available.
Similar articles
-
αII Spectrin Forms a Periodic Cytoskeleton at the Axon Initial Segment and Is Required for Nervous System Function.J Neurosci. 2017 Nov 22;37(47):11311-11322. doi: 10.1523/JNEUROSCI.2112-17.2017. Epub 2017 Oct 16. J Neurosci. 2017. PMID: 29038240 Free PMC article.
-
betaIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG.J Cell Biol. 2007 Feb 12;176(4):509-19. doi: 10.1083/jcb.200610128. Epub 2007 Feb 5. J Cell Biol. 2007. PMID: 17283186 Free PMC article.
-
An αII Spectrin-Based Cytoskeleton Protects Large-Diameter Myelinated Axons from Degeneration.J Neurosci. 2017 Nov 22;37(47):11323-11334. doi: 10.1523/JNEUROSCI.2113-17.2017. Epub 2017 Oct 16. J Neurosci. 2017. PMID: 29038243 Free PMC article.
-
Cytoskeletal control of axon domain assembly and function.Curr Opin Neurobiol. 2016 Aug;39:116-21. doi: 10.1016/j.conb.2016.05.001. Epub 2016 May 18. Curr Opin Neurobiol. 2016. PMID: 27203619 Free PMC article. Review.
-
Axon initial segments: diverse and dynamic neuronal compartments.Curr Opin Neurobiol. 2014 Aug;27:96-102. doi: 10.1016/j.conb.2014.03.004. Epub 2014 Apr 3. Curr Opin Neurobiol. 2014. PMID: 24705243 Free PMC article. Review.
Cited by
-
The role of the membrane-associated periodic skeleton in axons.Cell Mol Life Sci. 2021 Jul;78(13):5371-5379. doi: 10.1007/s00018-021-03867-x. Epub 2021 Jun 3. Cell Mol Life Sci. 2021. PMID: 34085116 Free PMC article. Review.
-
Axon Initial Segments Are Required for Efficient Motor Neuron Axon Regeneration and Functional Recovery of Synapses.J Neurosci. 2022 Oct 26;42(43):8054-8065. doi: 10.1523/JNEUROSCI.1261-22.2022. Epub 2022 Sep 12. J Neurosci. 2022. PMID: 36096668 Free PMC article.
-
Giant ankyrin-G: a critical innovation in vertebrate evolution of fast and integrated neuronal signaling.Proc Natl Acad Sci U S A. 2015 Jan 27;112(4):957-64. doi: 10.1073/pnas.1416544112. Epub 2014 Dec 31. Proc Natl Acad Sci U S A. 2015. PMID: 25552556 Free PMC article.
-
Preferential targeting of Nav1.6 voltage-gated Na+ Channels to the axon initial segment during development.PLoS One. 2015 Apr 15;10(4):e0124397. doi: 10.1371/journal.pone.0124397. eCollection 2015. PLoS One. 2015. PMID: 25874799 Free PMC article.
-
Exchange Protein Directly Activated by cAMP (EPAC) Regulates Neuronal Polarization through Rap1B.J Neurosci. 2015 Aug 12;35(32):11315-29. doi: 10.1523/JNEUROSCI.3645-14.2015. J Neurosci. 2015. PMID: 26269639 Free PMC article.
References
-
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

