Inhibitor mediated protein degradation
- PMID: 22633414
- PMCID: PMC3361691
- DOI: 10.1016/j.chembiol.2012.04.008
Inhibitor mediated protein degradation
Abstract
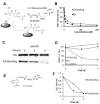
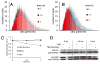
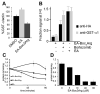
The discovery of drugs that cause the degradation of their target proteins has been largely serendipitous. Here we report that the tert-butyl carbamate-protected arginine (Boc(3)Arg) moiety provides a general strategy for the design of degradation-inducing inhibitors. The covalent inactivators ethacrynic acid and thiobenzofurazan cause the specific degradation of glutathione-S-transferase when linked to Boc(3)Arg. Similarly, the degradation of dihydrofolate reductase is induced when cells are treated with the noncovalent inhibitor trimethoprim linked to Boc(3)Arg. Degradation is rapid and robust, with 30%-80% of these abundant target proteins consumed within 1.3-5 hr. The proteasome is required for Boc(3)Arg-mediated degradation, but ATP is not necessary and the ubiquitin pathways do not appear to be involved. These results suggest that the Boc(3)Arg moiety may provide a general strategy to construct inhibitors that induce targeted protein degradation.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



References
-
- Aherne A, Kennan A, Kenna PF, McNally N, Lloyd DG, Alberts IL, Kiang AS, Humphries MM, Ayuso C, Engel PC, et al. On the molecular pathology of neurodegeneration in IMPDH1-based retinitis pigmentosa. Hum Mol Genet. 2004;13:641–650. - PubMed
-
- Asher G, Reuven N, Shaul Y. 20S proteasomes and protein degradation “by default”. Bioessays. 2006;28:844–849. - PubMed
-
- Bernier V, Lagace M, Bichet DG, Bouvier M. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab. 2004;15:222–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

