Transsynaptic control of presynaptic Ca²⁺ influx achieves homeostatic potentiation of neurotransmitter release
- PMID: 22633807
- PMCID: PMC4367479
- DOI: 10.1016/j.cub.2012.04.018
Transsynaptic control of presynaptic Ca²⁺ influx achieves homeostatic potentiation of neurotransmitter release
Abstract
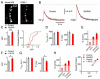
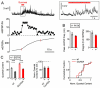
Given the complexity of the nervous system and its capacity for change, it is remarkable that robust, reproducible neural function and animal behavior can be achieved. It is now apparent that homeostatic signaling systems have evolved to stabilize neural function. At the neuromuscular junction (NMJ) of organisms ranging from Drosophila to human, inhibition of postsynaptic neurotransmitter receptor function causes a homeostatic increase in presynaptic release that precisely restores postsynaptic excitation. Here we address what occurs within the presynaptic terminal to achieve homeostatic potentiation of release at the Drosophila NMJ. By imaging presynaptic Ca(2+) transients evoked by single action potentials, we reveal a retrograde, transsynaptic modulation of presynaptic Ca(2+) influx that is sufficient to account for the rapid induction and sustained expression of the homeostatic change in vesicle release. We show that the homeostatic increase in Ca(2+) influx and release is blocked by a point mutation in the presynaptic CaV2.1 channel, demonstrating that the modulation of presynaptic Ca(2+) influx through this channel is causally required for homeostatic potentiation of release. Together with additional analyses, we establish that retrograde, transsynaptic modulation of presynaptic Ca(2+) influx through CaV2.1 channels is a key factor underlying the homeostatic regulation of neurotransmitter release.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

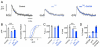
Similar articles
-
Endogenous Tagging Reveals Differential Regulation of Ca2+ Channels at Single Active Zones during Presynaptic Homeostatic Potentiation and Depression.J Neurosci. 2019 Mar 27;39(13):2416-2429. doi: 10.1523/JNEUROSCI.3068-18.2019. Epub 2019 Jan 28. J Neurosci. 2019. PMID: 30692227 Free PMC article.
-
Homeostatic synaptic depression is achieved through a regulated decrease in presynaptic calcium channel abundance.Elife. 2015 Apr 17;4:e05473. doi: 10.7554/eLife.05473. Elife. 2015. PMID: 25884248 Free PMC article.
-
α-Neurexins Together with α2δ-1 Auxiliary Subunits Regulate Ca2+ Influx through Cav2.1 Channels.J Neurosci. 2018 Sep 19;38(38):8277-8294. doi: 10.1523/JNEUROSCI.0511-18.2018. Epub 2018 Aug 13. J Neurosci. 2018. PMID: 30104341 Free PMC article.
-
Homeostatic control of presynaptic neurotransmitter release.Annu Rev Physiol. 2015;77:251-70. doi: 10.1146/annurev-physiol-021014-071740. Epub 2014 Nov 5. Annu Rev Physiol. 2015. PMID: 25386989 Review.
-
Homeostatic plasticity at the Drosophila neuromuscular junction.Neuropharmacology. 2014 Mar;78:63-74. doi: 10.1016/j.neuropharm.2013.06.015. Epub 2013 Jun 24. Neuropharmacology. 2014. PMID: 23806804 Free PMC article. Review.
Cited by
-
Homeostatic Plasticity of the Mammalian Neuromuscular Junction.Adv Neurobiol. 2022;28:111-130. doi: 10.1007/978-3-031-07167-6_5. Adv Neurobiol. 2022. PMID: 36066823
-
The calcineurin regulator Sarah enables distinct forms of homeostatic plasticity at the Drosophila neuromuscular junction.Front Synaptic Neurosci. 2023 Jan 4;14:1033743. doi: 10.3389/fnsyn.2022.1033743. eCollection 2022. Front Synaptic Neurosci. 2023. PMID: 36685082 Free PMC article.
-
Homeostatic signaling and the stabilization of neural function.Neuron. 2013 Oct 30;80(3):718-28. doi: 10.1016/j.neuron.2013.09.044. Neuron. 2013. PMID: 24183022 Free PMC article. Review.
-
Reactive oxygen species regulate activity-dependent neuronal plasticity in Drosophila.Elife. 2018 Dec 17;7:e39393. doi: 10.7554/eLife.39393. Elife. 2018. PMID: 30540251 Free PMC article.
-
Cul3 and insomniac are required for rapid ubiquitination of postsynaptic targets and retrograde homeostatic signaling.Nat Commun. 2019 Jul 5;10(1):2998. doi: 10.1038/s41467-019-10992-6. Nat Commun. 2019. PMID: 31278365 Free PMC article.
References
-
- Marder E, Goaillard J-M. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. - PubMed
-
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. - PubMed
-
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu. Rev. Neurosci. 2006;29:307–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

