Light-controlled synthetic gene circuits
- PMID: 22633822
- PMCID: PMC3424386
- DOI: 10.1016/j.cbpa.2012.04.010
Light-controlled synthetic gene circuits
Abstract
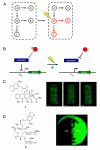
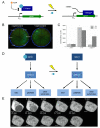
Highly complex synthetic gene circuits have been engineered in living organisms to develop systems with new biological properties. A precise trigger to activate or deactivate these complex systems is desired in order to tightly control different parts of a synthetic or natural network. Light represents an excellent tool to achieve this goal as it can be regulated in timing, location, intensity, and wavelength, which allows for precise spatiotemporal control over genetic circuits. Recently, light has been used as a trigger to control the biological function of small molecules, oligonucleotides, and proteins involved as parts in gene circuits. Light activation has enabled the construction of unique systems in living organisms such as band-pass filters and edge-detectors in bacterial cells. Additionally, light also allows for the regulation of intermediate steps of complex dynamic pathways in mammalian cells such as those involved in kinase networks. Herein we describe recent advancements in the area of light-controlled synthetic networks.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- Agapakis CM, Silver PA. Synthetic biology: exploring and exploiting genetic modularity through the design of novel biological networks. Mol Biosyst. 2009;5:704–713. - PubMed
-
- Tanouchi Y, Pai A, You L. Decoding biological principles using gene circuits. Mol Biosyst. 2009;5:695–703. - PubMed
-
- Weber W, Fussenegger M. Molecular diversity--the toolbox for synthetic gene switches and networks. Curr Opin Chem Biol. 2011;15:414–420. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

