Phosphatidylinositol 4-kinases: hostages harnessed to build panviral replication platforms
- PMID: 22633842
- PMCID: PMC3389303
- DOI: 10.1016/j.tibs.2012.03.004
Phosphatidylinositol 4-kinases: hostages harnessed to build panviral replication platforms
Abstract
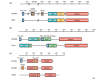
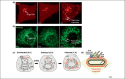
Several RNA viruses have recently been shown to hijack members of the host phosphatidylinositol (PtdIns) 4-kinase (PI4K) family of enzymes. They use PI4K to generate membranes enriched in phosphatidylinositide 4-phosphate (PtdIns4P or PI4P) lipids, which can be used as replication platforms. Viral replication machinery is assembled on these platforms as a supramolecular complex and PtdIns4P lipids regulate viral RNA synthesis. This article highlights these recent studies on the regulation of viral RNA synthesis by PtdIns4P lipids. It explores the potential mechanisms by which PtdIns4P lipids can contribute to viral replication and discusses the therapeutic potential of developing antiviral molecules that target host PI4Ks as a form of panviral therapy.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- den Boon J.A., Ahlquist P. Organelle-like membrane compartmentalization of positive-strand RNA virus replication factories. Annu. Rev. Microbiol. 2010;64:241–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

