Evaluation of substrate and inhibitor binding to yeast and human isoprenylcysteine carboxyl methyltransferases (Icmts) using biotinylated benzophenone-containing photoaffinity probes
- PMID: 22634004
- PMCID: PMC3718205
- DOI: 10.1016/j.bbrc.2012.05.089
Evaluation of substrate and inhibitor binding to yeast and human isoprenylcysteine carboxyl methyltransferases (Icmts) using biotinylated benzophenone-containing photoaffinity probes
Abstract
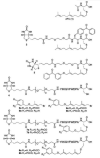
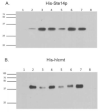
Isoprenylcysteine carboxyl methyltransferases (Icmts) are a class of integral membrane protein methyltransferases localized to the endoplasmic reticulum (ER) membrane in eukaryotes. The Icmts from human (hIcmt) and Saccharomyces cerevisiae (Ste14p) catalyze the α-carboxyl methyl esterification step in the post-translational processing of CaaX proteins, including the yeast a-factor mating pheromones and both human and yeast Ras proteins. Herein, we evaluated synthetic analogs of two well-characterized Icmt substrates, N-acetyl-S-farnesyl-L-cysteine (AFC) and the yeast a-factor peptide mating pheromone, that contain photoactive benzophenone moieties in either the lipid or peptide portion of the molecule. The AFC based-compounds were substrates for both hIcmt and Ste14p, whereas the a-factor analogs were only substrates for Ste14p. However, the a-factor analogs were found to be micromolar inhibitors of hIcmt. Together, these data suggest that the Icmt substrate binding site is dependent upon features in both the isoprenyl moiety and upstream amino acid composition. Furthermore, these data suggest that hIcmt and Ste14p have overlapping, yet distinct, substrate specificities. Photocrosslinking and neutravidin-agarose capture experiments with these analogs revealed that both hIcmt and Ste14p were specifically photolabeled to varying degrees with all of the compounds tested. Our data suggest that these analogs will be useful for the future identification of the Icmt substrate binding sites.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
References
-
- Hrycyna CA, Clarke S. Modification of eukaryotic signaling proteins by C-terminal methylation reactions. Pharmacol Ther. 1993;59:281–300. - PubMed
-
- Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. - PubMed
-
- Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. - PubMed
-
- Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. - PubMed
-
- Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

