Identification of radial glia-like cells in the adult mouse olfactory bulb
- PMID: 22634209
- PMCID: PMC3402915
- DOI: 10.1016/j.expneurol.2012.05.012
Identification of radial glia-like cells in the adult mouse olfactory bulb
Abstract
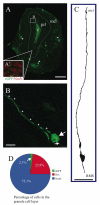
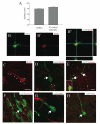
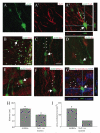
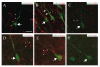
Immature neurons migrate tangentially within the rostral migratory stream (RMS) to the adult olfactory bulb (OB), then radially to their final positions as granule and periglomerular neurons; the controls over this transition are not well understood. Using adult transgenic mice with the human GFAP promoter driving expression of enhanced GFP, we identified a population of radial glia-like cells that we term adult olfactory radial glia-like cells (AORGs). AORGs have large, round somas and simple, radially oriented processes. Confocal reconstructions indicate that AORGs variably express typical radial glial markers, only rarely express mouse GFAP, and do not express astroglial, oligodendroglial, neuronal, or tanycyte markers. Electron microscopy provides further supporting evidence that AORGs are not immature neurons. Developmental analyses indicate that AORGs are present as early as P1, and are generated through adulthood. Tracing studies show that AORGs are not born in the SVZa, suggesting that they are born either in the RMS or the OB. Migrating immature neurons from the adult SVZa are closely apposed to AORGs during radial migration in vivo and in vitro. Taken together, these data indicate a newly-identified population of radial glia-like cells in the adult OB that might function uniquely in neuronal radial migration during adult OB neurogenesis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Intrinsic Neuronal Activity during Migration Controls the Recruitment of Specific Interneuron Subtypes in the Postnatal Mouse Olfactory Bulb.J Neurosci. 2021 Mar 24;41(12):2630-2644. doi: 10.1523/JNEUROSCI.1960-20.2021. Epub 2021 Feb 3. J Neurosci. 2021. PMID: 33536198 Free PMC article.
-
Differential neurogenesis and gliogenesis by local and migrating neural stem cells in the olfactory bulb.Neurosci Res. 2002 Dec;44(4):467-73. doi: 10.1016/s0168-0102(02)00173-6. Neurosci Res. 2002. PMID: 12445634
-
Cell cycle length of olfactory bulb neuronal progenitors in the rostral migratory stream.Dev Dyn. 1998 Oct;213(2):220-7. doi: 10.1002/(SICI)1097-0177(199810)213:2<220::AID-AJA7>3.0.CO;2-I. Dev Dyn. 1998. PMID: 9786422
-
Extracellular signals controlling neuroblast migration in the postnatal brain.Adv Exp Med Biol. 2014;800:149-80. doi: 10.1007/978-94-007-7687-6_9. Adv Exp Med Biol. 2014. PMID: 24243105 Review.
-
The Role of Adult-Born Neurons in the Constantly Changing Olfactory Bulb Network.Neural Plast. 2016;2016:1614329. doi: 10.1155/2016/1614329. Epub 2015 Dec 29. Neural Plast. 2016. PMID: 26839709 Free PMC article. Review.
Cited by
-
Clonal Mapping of Astrocytes in the Olfactory Bulb and Rostral Migratory Stream.Cereb Cortex. 2017 Mar 1;27(3):2195-2209. doi: 10.1093/cercor/bhw071. Cereb Cortex. 2017. PMID: 27001681 Free PMC article.
-
The extracellular matrix glycoprotein tenascin-R affects adult but not developmental neurogenesis in the olfactory bulb.J Neurosci. 2013 Jun 19;33(25):10324-39. doi: 10.1523/JNEUROSCI.5728-12.2013. J Neurosci. 2013. PMID: 23785146 Free PMC article.
-
Glial cells in the mammalian olfactory bulb.Front Cell Neurosci. 2024 Jul 16;18:1426094. doi: 10.3389/fncel.2024.1426094. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39081666 Free PMC article. Review.
References
-
- Alvarez-Buylla A, Buskirk DR, Nottebohm F. Monoclonal antibody reveals radial glia in adult avian brain. Journal of Comparative Neurology. 1987;264:159–170. - PubMed
-
- Alves JA, Barone P, Engelender S, Froes MM, Menezes JR. Initial stages of radial glia astrocytic transformation in the early postnatal anterior subventricular zone. Journal of Neurobiology. 2002;52:251–265. - PubMed
-
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. - PubMed
-
- Au E, Roskams AJ. Olfactory ensheathing cells of the lamina propria in vivo and in vitro. Glia. 2003;41:224–236. - PubMed
-
- Bailey MS, Puche AC, Shipley MT. Development of the olfactory bulb, evidence for glia-neuron interactions in glomerular formation. Journal of Comparative Neurology. 1999;415:423–448. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

