IgG-Fc glycoengineering in non-mammalian expression hosts
- PMID: 22634260
- PMCID: PMC3442181
- DOI: 10.1016/j.abb.2012.05.011
IgG-Fc glycoengineering in non-mammalian expression hosts
Abstract
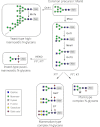
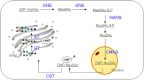
The remarkable success of therapeutic applications of immunoglobulin G (IgG) in form of monoclonal antibodies and pooled immunoglobulin G preparations has directed attention to this class of glycoproteins. It is commonly appreciated that oligosaccharides attached to the Fc-region play a critical role in the biological activity of IgGs. Thus, glycosylation has been a focus of interest for many scientists and the biopharmaceutical industry and expression hosts have been engineered in order to optimize antibody products. In this review we focus on efforts towards a targeted manipulation of IgG-Fc N-glycans using non-mammalian expression hosts, i.e. yeast, insect cells and plants. Current achievements in generating human-like N-glycan structures will be presented and recent data on the molecular mechanisms that might explain how these potent drugs mediate in vivo activities will be discussed.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Immune recruitment or suppression by glycan engineering of endogenous and therapeutic antibodies.Biochim Biophys Acta. 2016 Aug;1860(8):1655-68. doi: 10.1016/j.bbagen.2016.04.016. Epub 2016 Apr 20. Biochim Biophys Acta. 2016. PMID: 27105835 Free PMC article. Review.
-
Multi-Angle Effector Function Analysis of Human Monoclonal IgG Glycovariants.PLoS One. 2015 Dec 11;10(12):e0143520. doi: 10.1371/journal.pone.0143520. eCollection 2015. PLoS One. 2015. PMID: 26657484 Free PMC article.
-
Structural characterization of anti-inflammatory immunoglobulin G Fc proteins.J Mol Biol. 2014 Sep 9;426(18):3166-3179. doi: 10.1016/j.jmb.2014.07.006. Epub 2014 Jul 15. J Mol Biol. 2014. PMID: 25036289 Free PMC article.
-
Modulating IgG effector function by Fc glycan engineering.Proc Natl Acad Sci U S A. 2017 Mar 28;114(13):3485-3490. doi: 10.1073/pnas.1702173114. Epub 2017 Mar 13. Proc Natl Acad Sci U S A. 2017. PMID: 28289219 Free PMC article.
-
Glycosylation of plant produced human antibodies.Hum Antibodies. 2015 Dec 23;23(3-4):45-8. doi: 10.3233/HAB-150283. Hum Antibodies. 2015. PMID: 27472861 Review.
Cited by
-
Long-Term Culturing of FreeStyle 293-F Cells Affects Immunoglobulin G Glycome Composition.Biomolecules. 2023 Aug 14;13(8):1245. doi: 10.3390/biom13081245. Biomolecules. 2023. PMID: 37627310 Free PMC article.
-
Oligomerization status influences subcellular deposition and glycosylation of recombinant butyrylcholinesterase in Nicotiana benthamiana.Plant Biotechnol J. 2014 Sep;12(7):832-9. doi: 10.1111/pbi.12184. Epub 2014 Mar 11. Plant Biotechnol J. 2014. PMID: 24618259 Free PMC article.
-
Plant-derived Durvalumab variants show efficient PD-1/PD-L1 blockade and therapeutically favourable FcR binding.Plant Biotechnol J. 2024 May;22(5):1224-1237. doi: 10.1111/pbi.14260. Epub 2023 Dec 4. Plant Biotechnol J. 2024. PMID: 38050338 Free PMC article.
-
Generation and analysis of novel plant-derived antibody-based therapeutic molecules against West Nile virus.PLoS One. 2014 Mar 27;9(3):e93541. doi: 10.1371/journal.pone.0093541. eCollection 2014. PLoS One. 2014. PMID: 24675995 Free PMC article.
-
Strategies for Glycoengineering Therapeutic Proteins.Front Chem. 2022 Apr 13;10:863118. doi: 10.3389/fchem.2022.863118. eCollection 2022. Front Chem. 2022. PMID: 35494652 Free PMC article. Review.
References
-
- R. Apweiler, H. Hermjakob, N. Sharon, Biochimica et Biophysica Acta (BBA) – General Subjects 1473 (1999) 4–8. - PubMed
-
- R. Jefferis, Archieves of Biochemistry and Biophysics, 2012. - PubMed
-
- Arnold J.N., Wormald M.R., Sim R.B., Rudd P.M., Dwek R.A. Annual Review of Immunology. 2007;25:21–50. - PubMed
-
- Nezlin R. Academic Press; New York: 1998. The Immunoglobulins: Structure and Function.
-
- Arnold J.N., Radcliffe C.M., Wormald M.R., Royle L., Harvey D.J., Crispin M., Dwek R.A., Sim R.B., Rudd P.M. The Journal of Immunology. 2004;173:6831–6840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

