Abnormal PfEMP1/knob display on Plasmodium falciparum-infected erythrocytes containing hemoglobin variants: fresh insights into malaria pathogenesis and protection
- PMID: 22634344
- PMCID: PMC3396718
- DOI: 10.1016/j.micinf.2012.05.006
Abnormal PfEMP1/knob display on Plasmodium falciparum-infected erythrocytes containing hemoglobin variants: fresh insights into malaria pathogenesis and protection
Abstract
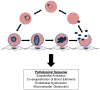
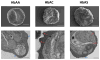
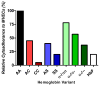
Hemoglobin (Hb) variants are associated with reduced risk of life-threatening Plasmodium falciparum malaria syndromes, including cerebral malaria and severe malarial anemia. Despite decades of research, the mechanisms by which common Hb variants - sickle HbS, HbC, α-thalassemia, fetal HbF - protect African children against severe and fatal malaria have not been fully elucidated. In vitro experimental and epidemiological data have long suggested that Hb variants do not confer malaria protection by restricting the growth of parasites in red blood cells (RBCs). Recently, four Hb variants were found to impair cytoadherence, the binding of P. falciparum-infected RBCs (PfRBCs) to microvascular endothelial cells (MVECs), a centrally important event in both parasite survival and malaria pathogenesis in humans. Impaired cytoadherence is associated with abnormal display of P. falciparum erythrocyte membrane protein 1 (PfEMP1), the parasite's major cytoadherence ligand and virulence factor, on the surface of host RBCs. We propose a model in which Hb variants allow parasites to display relatively low levels of PfEMP1, sufficient for sequestering PfRBCs in microvessels and avoiding their clearance from the bloodstream by the spleen. By preventing the display of high levels of PfEMP1, Hb variants may weaken the binding of PfRBCs to MVECs, compromising their ability to activate endothelium and initiate the downstream microvascular events that drive the pathogenesis of malaria.
Published by Elsevier Masson SAS.
Figures

References
-
- WHO, Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. - PubMed
-
- Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

