Adenosine signaling in normal and sickle erythrocytes and beyond
- PMID: 22634345
- PMCID: PMC3842013
- DOI: 10.1016/j.micinf.2012.05.005
Adenosine signaling in normal and sickle erythrocytes and beyond
Abstract
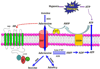
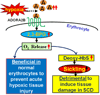
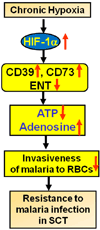
Sickle cell disease (SCD) is a debilitating hemolytic genetic disorder with high morbidity and mortality affecting millions of individuals worldwide. Although SCD was discovered more than a century ago, no effective mechanism-based prevention and treatment are available due to poorly understood molecular basis of sickling, the fundamental pathogenic process of the disease. SCD patients constantly face hypoxia. One of the best-known signaling molecules to be induced under hypoxic conditions is adenosine. Recent studies demonstrate that hypoxia-mediated elevated adenosine signaling plays an important role in normal erythrocyte physiology. In contrast, elevated adenosine signaling contributes to sickling and multiple life threatening complications including tissue damage, pulmonary dysfunction and priapism. Here, we summarize recent research on the role of adenosine signaling in normal and sickle erythrocytes, progression of the disease and therapeutic implications. In normal erythrocytes, both genetic and pharmacological studies demonstrate that adenosine can enhance 2,3-bisphosphoglycerate (2,3-BPG) production via A(2B) receptor (ADORA2B) activation, suggesting that elevated adenosine has an unrecognized role in normal erythrocytes to promote O(2) release and prevent acute ischemic tissue injury. However, in sickle erythrocytes, the beneficial role of excessive adenosine-mediated 2,3-BPG induction becomes detrimental by promoting deoxygenation, polymerization of sickle hemoglobin and subsequent sickling. Additionally, adenosine signaling via the A(2A) receptor (ADORA2A) on invariant natural killer T (iNKT) cells inhibits iNKT cell activation and attenuates pulmonary dysfunction in SCD mice. Finally, elevated adenosine coupled with ADORA2BR activation is responsible for priapism, a dangerous complication seen in SCD. Overall, the research reviewed here reveals a differential role of elevated adenosine in normal erythrocytes, sickle erythrocytes, iNK cells and progression of disease. Thus, adenosine signaling represents a potentially important therapeutic target for the treatment and prevention of disease.
Copyright © 2012 Institut Pasteur. Published by Elsevier Masson SAS. All rights reserved.
Figures

Similar articles
-
Elevated ecto-5'-nucleotidase: a missing pathogenic factor and new therapeutic target for sickle cell disease.Blood Adv. 2018 Aug 14;2(15):1957-1968. doi: 10.1182/bloodadvances.2018015784. Blood Adv. 2018. PMID: 30097462 Free PMC article.
-
Elevated adenosine signaling via adenosine A2B receptor induces normal and sickle erythrocyte sphingosine kinase 1 activity.Blood. 2015 Mar 5;125(10):1643-52. doi: 10.1182/blood-2014-08-595751. Epub 2015 Jan 13. Blood. 2015. PMID: 25587035 Free PMC article.
-
Detrimental effects of adenosine signaling in sickle cell disease.Nat Med. 2011 Jan;17(1):79-86. doi: 10.1038/nm.2280. Epub 2010 Dec 19. Nat Med. 2011. PMID: 21170046 Free PMC article.
-
Erythrocyte adaptive metabolic reprogramming under physiological and pathological hypoxia.Curr Opin Hematol. 2020 May;27(3):155-162. doi: 10.1097/MOH.0000000000000574. Curr Opin Hematol. 2020. PMID: 32141895 Free PMC article. Review.
-
Metabolomic and molecular insights into sickle cell disease and innovative therapies.Blood Adv. 2019 Apr 23;3(8):1347-1355. doi: 10.1182/bloodadvances.2018030619. Blood Adv. 2019. PMID: 31015210 Free PMC article. Review.
Cited by
-
A narrative review of the therapeutic and remedial prospects of cannabidiol with emphasis on neurological and neuropsychiatric disorders.J Cannabis Res. 2024 Mar 18;6(1):14. doi: 10.1186/s42238-024-00222-2. J Cannabis Res. 2024. PMID: 38494488 Free PMC article. Review.
-
Minireview: Multiomic candidate biomarkers for clinical manifestations of sickle cell severity: Early steps to precision medicine.Exp Biol Med (Maywood). 2016 Apr;241(7):772-81. doi: 10.1177/1535370216640150. Epub 2016 Mar 27. Exp Biol Med (Maywood). 2016. PMID: 27022133 Free PMC article. Review.
-
Interventions for treating intrahepatic cholestasis in people with sickle cell disease.Cochrane Database Syst Rev. 2020 Jun 22;6(6):CD010985. doi: 10.1002/14651858.CD010985.pub4. Cochrane Database Syst Rev. 2020. PMID: 32567054 Free PMC article.
-
Sustained Elevated Adenosine via ADORA2B Promotes Chronic Pain through Neuro-immune Interaction.Cell Rep. 2016 Jun 28;16(1):106-119. doi: 10.1016/j.celrep.2016.05.080. Epub 2016 Jun 16. Cell Rep. 2016. PMID: 27320922 Free PMC article.
-
Metabolism, homeostasis, and aging.Trends Endocrinol Metab. 2023 Mar;34(3):158-169. doi: 10.1016/j.tem.2023.01.003. Epub 2023 Jan 20. Trends Endocrinol Metab. 2023. PMID: 36681595 Free PMC article. Review.
References
-
- Madigan C, Malik P. Pathophysiology and therapy for haemoglobinopathies. Part I: sickle cell disease. Exp. Rev. Mol. Med. 2006;8:1–23. - PubMed
-
- Urbinati F, Madigan C, Malik P. Pathophysiology and therapy for haemoglobinopathies. Part II: thalassaemias. Exp. Rev. Mol. Med. 2006;8:1–26. - PubMed
-
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2011;376:2018–2031. - PubMed
-
- Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088–3098. - PMC - PubMed
-
- Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N. Engl. J. Med. 2008;359:2254–2265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

