Methodologically rigorous clinical research
- PMID: 22634695
- PMCID: PMC3531969
- DOI: 10.1097/PRS.0b013e31824eccb7
Methodologically rigorous clinical research
Abstract
Background: Rigorous methodology increases the quality of clinical research by encouraging freedom from the biases inherent in clinical studies. As randomized controlled studies (clinical trial design) are rarely applicable to surgical research, the authors address the commonly used observational study designs and methodologies by presenting guidelines for rigor.
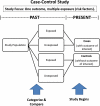
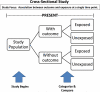
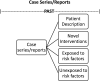
Methods: The authors performed a review of study designs, including cohort, case-control, and cross-sectional studies and case series/reports, and biases and confounders of study design.
Results: Details about biases and confounders at each study stage, study characteristics, rigor checklists, and published literature examples for each study design are summarized and presented in this report.
Conclusions: For those surgeons interested in pursuing clinical research, mastery of the principles of methodologic rigor is imperative in the context of evidence-based medicine and widespread publication of clinical studies. Knowledge of the study designs and their appropriate application, and strict adherence to study design methods can provide high-quality evidence to serve as the basis for rational clinical decision-making.
Figures


References
-
- [July 1, 2011];Clinical Research: A National Call to Action (American Association of Medical Colleges and the American Medical Association) Anonymous. Available at: http://www.umassmed.edu/uploadedfiles/NationalCalltoAction.pdf.
-
- Timmermans S, Mauck A. The promises and pitfalls of evidence-based medicine. Health Aff (Millwood) 2005;24:18–28. - PubMed
-
- [July 1, 2011];Guide to Clinical Preventive Services (U.S. Preventive Services Task Force) Anonymous. Available at: http://odphp.osophs.dhhs.gov/pubs/guidecps/pcpstoc.htm.
-
- Brighton B, Bhandari M, Tornetta P, 3rd, Felson DT. Hierarchy of evidence: from case reports to randomized controlled trials. Clin Orthop Relat Res. 2003;(413):19–24. - PubMed
-
- Grimes DA, Schulz KF. Descriptive studies: what they can and cannot do. Lancet. 2002;359:145–149. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

