Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials
- PMID: 22634875
- PMCID: PMC3392187
- DOI: 10.1093/infdis/jis367
Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials
Abstract
Background: A recombinant canarypox vector expressing human immunodeficiency virus type 1 (HIV-1) Gag, Pro, and membrane-linked gp120 (vCP1521), combined with a bivalent gp120 protein boost (AIDSVAX B/E), provided modest protection against HIV-1 infection in a community-based population in Thailand (RV144 trial). No protection was observed in Thai injection drug users who received AIDSVAX B/E alone (Vax003 trial). We compared the neutralizing antibody response in these 2 trials.
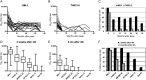
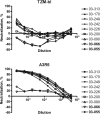
Methods: Neutralization was assessed with tier 1 and tier 2 strains of virus in TZM-bl and A3R5 cells.
Results: Neutralization of several tier 1 viruses was detected in both RV144 and Vax003. Peak titers were higher in Vax003 and waned rapidly in both trials. The response in RV144 was targeted in part to V3 of gp120.vCP1521 priming plus 2 boosts with gp120 protein was superior to 2 gp120 protein inoculations alone, confirming a priming effect for vCP1521. Sporadic weak neutralization of tier 2 viruses was detected only in Vax003 and A3R5 cells.
Conclusion: The results suggest either that weak neutralizing antibody responses can be partially protective against HIV-1 in low-risk heterosexual populations or that the modest efficacy seen in RV144 was mediated by other immune responses, either alone or in combination with neutralizing antibodies.
Figures



References
-
- Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Ann Rev Immunol. 2010;28:413–44. - PubMed
-
- Hoxie JA. Toward an antibody-based HIV-1 vaccine. Ann Rev Med. 2010;61:135–52. - PubMed
-
- Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med. 2009;15:866–70. - PubMed
-
- Shibata R, Igarashi T, Haigwood N, et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–10. - PubMed
-
- Mascola JR, Stiegler G, VanCott TC, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

