Ketamine alters the neurogenesis of rat cortical neural stem progenitor cells
- PMID: 22635046
- PMCID: PMC3507468
- DOI: 10.1097/CCM.0b013e318253563c
Ketamine alters the neurogenesis of rat cortical neural stem progenitor cells
Abstract
Objective: High doses or prolonged exposure to ketamine increase neuronal apoptosis in the developing brain, although effects on neural stem progenitor cells remain unexplored. This study investigated dose- and time-dependent responses to ketamine on cell death and neurogenesis in cultured rat fetal cortical neural stem progenitor cells.
Design: Laboratory-based study.
Setting: University research laboratory.
Subject: Sprague-Dawley rats.
Interventions: Neural stem progenitor cells were isolated from the cortex of Sprague-Dawley rat fetuses on embryonic day 17. In dose-response experiments, cultured neural stem progenitor cells were exposed to different concentrations of ketamine (0-100 µM) for 24 hrs. In time-course experiments, neural stem progenitor cells cultures were exposed to 10 µM ketamine for different durations (0-48 hrs).
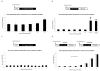
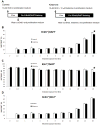
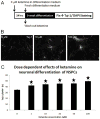
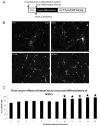
Measurements and main results: Apoptosis and necrosis in neural stem progenitor cells were assessed using activated caspase-3 immunostaining and lactate dehydrogenase assays, respectively. Proliferative changes in neural stem progenitor cells were detected using bromo-deoxyuridine incorporation and Ki67 immunostaining. Neuronal differentiation was assessed using Tuj-1 immunostaining. Cultured neural stem progenitor cells were resistant to apoptosis and necrosis following all concentrations and durations of ketamine exposure tested. Ketamine inhibited proliferation with decreased numbers of bromo-deoxyuridine-positive cells following ketamine exposure to 100 µM for 24 hrs (p<.005) or 10 µM for 48 hrs (p< .01), and reduced numbers of Ki67-positive cells following exposure to ketamine concentration>10 µM for 24 hrs (p<.001) or at 10 µM for 48 hrs (p<.01). Ketamine enhanced neuronal differentiation, with all ketamine concentrations increasing Tuj-1-positive neurons (p<.001) after 24-hrs of exposure. This also occurred with all exposures to 10 µM ketamine for >8 hrs (p<.001).
Conclusions: Clinically relevant concentrations of ketamine do not induce cell death in neural stem progenitor cells via apoptosis or necrosis. Ketamine alters the proliferation and increases the neuronal differentiation of neural stem progenitor cells isolated from the rat neocortex. These studies imply that ketamine exposure during fetal or neonatal life may alter neurogenesis and subsequent brain development.
Conflict of interest statement
The authors have not disclosed any potential conflicts of interest
Figures



Comment in
-
Neurotoxicity of ketamine: known unknowns.Crit Care Med. 2012 Aug;40(8):2518-9. doi: 10.1097/CCM.0b013e31825ae442. Crit Care Med. 2012. PMID: 22809932 No abstract available.
References
-
- Sinner B, Graf BM. Ketamine. Handb Exp Pharmacol. 2008;182:313–333. - PubMed
-
- Rappaport B, Mellon RD, Simone A, et al. Defining safe use of anesthesia in children. N Engl J Med. 2011;364(15):1387–1390. - PubMed
-
- Ikonomidou C, Bosch F, Miksa M, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–74. - PubMed
-
- Scallet AC, Schmued LC, Slikker W, Jr, et al. Developmental neurotoxicity of ketamine: morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol Sci. 2004;81(2):364–370. - PubMed
-
- Slikker W, Jr, Zou X, Hotchkiss CE, et al. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98(1):145–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

