A new in vivo model to test anti-tuberculosis drugs using fluorescence imaging
- PMID: 22635525
- PMCID: PMC3394442
- DOI: 10.1093/jac/dks161
A new in vivo model to test anti-tuberculosis drugs using fluorescence imaging
Abstract
Objectives: The current method for testing new drugs against tuberculosis in vivo is the enumeration of bacteria in organs by cfu assay. Owing to the slow growth rate of Mycobacterium tuberculosis (Mtb), these assays can take months to complete. Our aim was to develop a more efficient, fluorescence-based imaging assay to test new antibiotics in a mouse model using Mtb reporter strains.
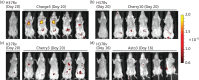
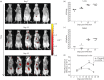
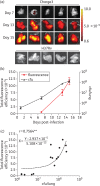
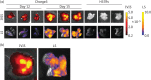
Methods: A commercial IVIS Kinetic® system and a custom-built laser scanning system with fluorescence molecular tomography (FMT) capability were used to detect fluorescent Mtb in living mice and lungs ex vivo. The resulting images were analysed and the fluorescence was correlated with data from cfu assays.
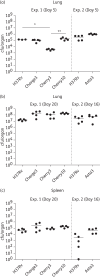
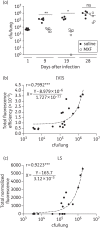
Results: We have shown that fluorescent Mtb can be visualized in the lungs of living mice at a detection limit of ∼8 × 10⁷ cfu/lung, whilst in lungs ex vivo a detection limit of ∼2 × 10⁵ cfu/lung was found. These numbers were comparable between the two imaging systems. Ex vivo lung fluorescence correlated to numbers of bacteria in tissue, and the effect of treatment of mice with the antibiotic moxifloxacin could be visualized and quantified after only 9 days through fluorescence measurements, and was confirmed by cfu assays.
Conclusions: We have developed a new and efficient method for anti-tuberculosis drug testing in vivo, based on fluorescent Mtb reporter strains. Using this method instead of, or together with, cfu assays will reduce the time required to assess the preclinical efficacy of new drugs in animal models and enhance the progress of these candidates into clinical trials against human tuberculosis.
Figures

References
-
- WHO. Tuberculosis. http://www.who.int/mediacentre/factsheets/fs104/en/index.html. (23 September 2011, date last accessed)
-
- Rowland R, McShane H. Tuberculosis vaccines in clinical trials. Expert Rev Vaccines. 2011;10:645–58. doi:10.1586/erv.11.28. - DOI - PMC - PubMed
-
- Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. doi:10.1001/jama.1994.03510330076038. - DOI - PubMed
-
- WHO. Map: Available Data on Anti-TB Drug Resistance, 2010. http://www.who.int/tb/challenges/mdr/drs_maps_feb2011.pdf. (23 September 2011, date last accessed)
-
- Dorhoi A, Reece ST, Kaufmann SH. For better or for worse: the immune response against Mycobacterium tuberculosis balances pathology and protection. Immunol Rev. 2011;240:235–51. doi:10.1111/j.1600-065X.2010.00994.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

