The continuing evolution of the Langendorff and ejecting murine heart: new advances in cardiac phenotyping
- PMID: 22636675
- PMCID: PMC3404701
- DOI: 10.1152/ajpheart.00333.2012
The continuing evolution of the Langendorff and ejecting murine heart: new advances in cardiac phenotyping
Abstract
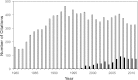
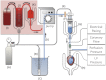
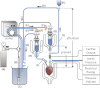
The isolated retrograde-perfused Langendorff heart and the isolated ejecting heart have, over many decades, resulted in fundamental discoveries that form the underpinnings of our current understanding of the biology and physiology of the heart. These two experimental methodologies have proven invaluable in studying pharmacological effects on myocardial function, metabolism, and vascular reactivity and in the investigation of clinically relevant disease states such as ischemia-reperfusion injury, diabetes, obesity, and heart failure. With the advent of the genomics era, the isolated mouse heart preparation has gained prominence as an ex vivo research tool for investigators studying the impact of gene modification in the intact heart. This review summarizes the historical development of the isolated heart and provides a practical guide for the establishment of the Langendorff and ejecting heart preparations with a particular emphasis on the murine heart. In addition, current applications and novel methods of recording cardiovascular parameters in the isolated heart preparation will be discussed. With continued advances in methodological recordings, the isolated mouse heart preparation will remain physiologically relevant for the foreseeable future, serving as an integral bridge between in vitro assays and in vivo approaches.
Figures

References
-
- Aasum E, Hafstad AD, Larsen TS. Changes in substrate metabolism in isolated mouse hearts following ischemia-reperfusion. Mol Cell Biochem 249: 97– 103, 2003 - PubMed
-
- Albritton E. Standard Values in Blood. Philadelphia: Saunders, 1952
-
- Assayag P, Charlemagne D, Marty I, de Leiris J, Lompre AM, Boucher F, Valere PE, Lortet S, Swynghedauw B, Besse S. Effects of sustained low-flow ischemia on myocardial function and calcium-regulating proteins in adult and senescent rat hearts. Cardiovasc Res 38: 169– 180, 1998 - PubMed
-
- Baker LC, London B, Choi BR, Koren G, Salama G. Enhanced dispersion of repolarization and refractoriness in transgenic mouse hearts promotes reentrant ventricular tachycardia. Circ Res 86: 396– 407, 2000 - PubMed
-
- Barr RL, Lopaschuk GD. Methodology for measuring in vitro/ex vivo cardiac energy metabolism. J Pharmacol Toxicol Methods 43: 141– 152, 2000 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

