FeoB2 Functions in magnetosome formation and oxidative stress protection in Magnetospirillum gryphiswaldense strain MSR-1
- PMID: 22636767
- PMCID: PMC3416554
- DOI: 10.1128/JB.00382-12
FeoB2 Functions in magnetosome formation and oxidative stress protection in Magnetospirillum gryphiswaldense strain MSR-1
Abstract
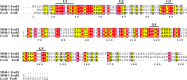
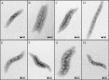
Magnetotactic bacteria (MTB) synthesize unique organelles, the magnetosomes, which are intracellular nanometer-sized, membrane-enveloped magnetite. The biomineralization of magnetosomes involves the uptake of large amounts of iron. However, the iron metabolism of MTB is not well understood. The genome of the magnetotactic bacterium Magnetospirillum gryphiswaldense strain MSR-1 contains two ferrous iron transport genes, feoB1 and feoB2. The FeoB1 protein was reported to be responsible mainly for the transport of ferrous iron and to play an accessory role in magnetosome formation. To determine the role of feoB2, we constructed an feoB2 deletion mutant (MSR-1 ΔfeoB2) and an feoB1 feoB2 double deletion mutant (MSR-1 NfeoB). The single feoB2 mutation did not affect magnetite crystal biomineralization. MSR-1 NfeoB had a significantly lower average magnetosome number per cell (∼65%) than MSR-1 ΔfeoB1, indicating that FeoB2 plays a role in magnetosome formation when the feoB1 gene is deleted. Our findings showed that FeoB1 has a greater ferrous iron transport ability than FeoB2 and revealed the differential roles of FeoB1 and FeoB2 in MSR-1 iron metabolism. Interestingly, compared to the wild type, the feoB mutants showed increased sensitivity to oxidative stress and lower activities of the enzymes superoxide dismutase and catalase, indicating that the FeoB proteins help protect bacterial cells from oxidative stress.
Figures


References
-
- Andrews SC, Robinson AK, Rodriguez-Quinones F. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215–237 - PubMed
-
- Bazylinski DA, Frankel RB. 2004. Magnetosome formation in prokaryotes. Nat. Rev. Microbiol. 2:217–230 - PubMed
-
- Calugay RJ, Miyashita H, Okamura Y, Matsunaga T. 2003. Siderophore production by the magnetic bacterium Magnetospirillum magneticum AMB-1. FEMS Microbiol. Lett. 218:371–375 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

