Growth phase-dependent modulation of Rgg binding specificity in Streptococcus pyogenes
- PMID: 22636768
- PMCID: PMC3416553
- DOI: 10.1128/JB.06709-11
Growth phase-dependent modulation of Rgg binding specificity in Streptococcus pyogenes
Abstract
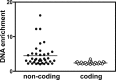
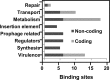
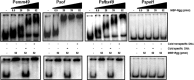
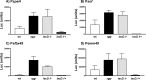
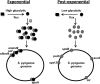
Streptococcus pyogenes Rgg is a transcriptional regulator that interacts with the cofactor LacD.1 to control growth phase-dependent expression of genes, including speB, which encodes a secreted cysteine protease. LacD.1 is thought to interact with Rgg when glycolytic intermediates are abundant in a manner that prevents Rgg-mediated activation of speB expression via binding to the promoter region. When the intermediates diminish, LacD.1 dissociates from Rgg and binds to the speB promoter to activate expression. The purpose of this study was to determine if Rgg bound to chromatin during the exponential phase of growth and, if so, to identify the binding sites. Rgg bound to 62 chromosomal sites, as determined by chromatin immunoprecipitation coupled with DNA microarrays. Thirty-eight were within noncoding DNA, including sites upstream of the genes encoding the M protein (M49), serum opacity factor (SOF), fibronectin-binding protein (SfbX49), and a prophage-encoded superantigen, SpeH. Each of these sites contained a promoter that was regulated by Rgg, as determined with transcriptional fusion assays. Purified Rgg also bound to the promoter regions of emm49, sof, and sfbX49 in vitro. Results obtained with a lacD.1 mutant showed that both LacD.1 and Rgg were necessary for the repression of emm49, sof, sfbX49, and speH expression. Overall, the results indicated that the DNA binding specificity of Rgg is responsive to environmental changes in a LacD.1-dependent manner and that Rgg and LacD.1 directly control virulence gene expression in the exponential phase of growth.
Figures
References
-
- Banks DJ, Beres SB, Musser JM. 2002. The fundamental contribution of phages to GAS evolution, genome diversification and strain emergence. Trends Microbiol. 10:515–521 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

