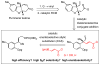
Copper-catalyzed enantioselective allylic substitution with readily accessible carbonyl- and acetal-containing vinylboron reagents
- PMID: 22639429
- PMCID: PMC3406181
- DOI: 10.1002/anie.201202856
Copper-catalyzed enantioselective allylic substitution with readily accessible carbonyl- and acetal-containing vinylboron reagents
Figures




References
-
-
For reviews of catalytic enantioselective allylic substitution (EAS) reactions with “hard” organometallic nucleophiles, see: Hoveyda AH, Hird AW, Kacprzynski MA. Chem Commun. 2004:1779–1785.Yorimitsu H, Oshima K. Angew Chem Int Ed. 2005;44:4435–4439.Harutyunyan SR, den Hartog T, Geurts K, Minnaard AJ, Feringa BL. Chem Rev. 2008;108:2824–2852.Alexakis A, Bäckvall JE, Krause N, Pàmies O, Diéguez M. Chem Rev. 2008;108:2796–2823.
-
-
- Kacprzynski MA, May TL, Kazane SA, Hoveyda AH. Angew Chem Int Ed. 2007;46:4554–4558. - PubMed
- Selim KB, Yamada K-i, Tomioka K. Chem Commun. 2008:5140–5142. - PubMed
- Falciola CA, Alexakis A. Chem Eur J. 2008;14:10615–10627. - PubMed
- Selim KB, Matsumoto Y, Yamada K-i, Tomioka K. Angew Chem Int Ed. 2009;48:8733–8735. - PubMed
- Polet D, Rathgeb X, Falciola CA, Langlois JB, Hajjaji SE, Alexakis A. Chem Eur J. 2009;15:1205–1216. - PubMed
- Gao F, Lee Y, Mandai K, Hoveyda AH. Angew Chem Int Ed. 2010;49:8370–8374. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

