Crosstalk between Phospholipase D and Sphingosine Kinase in Plant Stress Signaling
- PMID: 22639650
- PMCID: PMC3355621
- DOI: 10.3389/fpls.2012.00051
Crosstalk between Phospholipase D and Sphingosine Kinase in Plant Stress Signaling
Abstract
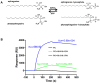
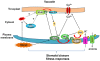
The activation of phospholipase D (PLD) produces phosphatidic acid (PA), whereas plant sphingosine kinase (SPHK) phosphorylates long-chain bases to generate long-chain base-1-phosphates such as phytosphingosine-1-phosphate (phyto-S1P). PA and phyto-S1P have been identified as lipid messengers. Recent studies have shown that PA interacts directly with SPHKs in Arabidopsis, and that the interaction promotes SPHK activity. However, SPHK and phyto-S1P act upstream of PLDα1 and PA in the stomatal response to abscisic acid (ABA). These findings indicate that SPHK/phyto-S1P and PLD/PA are co-dependent in the amplification of lipid messengers, and that crosstalk between the sphingolipid- and phospholipid-mediated signaling pathways may play important roles in plant stress signaling.
Keywords: abscisic acid; lipid signaling; phosphatidic acid; phospholipase D; phytosphingosine; sphingosine kinase.
Figures

Similar articles
-
Connections between sphingosine kinase and phospholipase D in the abscisic acid signaling pathway in Arabidopsis.J Biol Chem. 2012 Mar 9;287(11):8286-96. doi: 10.1074/jbc.M111.274274. Epub 2012 Jan 24. J Biol Chem. 2012. PMID: 22275366 Free PMC article.
-
Stomatal closure induced by phytosphingosine-1-phosphate and sphingosine-1-phosphate depends on nitric oxide and pH of guard cells in Pisum sativum.Planta. 2016 Oct;244(4):831-41. doi: 10.1007/s00425-016-2545-z. Epub 2016 May 27. Planta. 2016. PMID: 27233507
-
Phosphatidic acid binds and stimulates Arabidopsis sphingosine kinases.J Biol Chem. 2011 Apr 15;286(15):13336-45. doi: 10.1074/jbc.M110.190892. Epub 2011 Feb 17. J Biol Chem. 2011. PMID: 21330371 Free PMC article.
-
Sphingosine kinase, sphingosine-1-phosphate, and apoptosis.Biochim Biophys Acta. 2002 Dec 30;1585(2-3):193-201. doi: 10.1016/s1388-1981(02)00341-4. Biochim Biophys Acta. 2002. PMID: 12531554 Review.
-
The Crosstalk between FcεRI and Sphingosine Signaling in Allergic Inflammation.Int J Mol Sci. 2022 Nov 11;23(22):13892. doi: 10.3390/ijms232213892. Int J Mol Sci. 2022. PMID: 36430378 Free PMC article. Review.
Cited by
-
Phosphatidic acid produced by phospholipase D promotes RNA replication of a plant RNA virus.PLoS Pathog. 2015 May 28;11(5):e1004909. doi: 10.1371/journal.ppat.1004909. eCollection 2015 May. PLoS Pathog. 2015. PMID: 26020241 Free PMC article.
-
Cell Membrane Features as Potential Breeding Targets to Improve Cold Germination Ability of Seeds.Plants (Basel). 2022 Dec 6;11(23):3400. doi: 10.3390/plants11233400. Plants (Basel). 2022. PMID: 36501439 Free PMC article. Review.
-
The guard cell metabolome: functions in stomatal movement and global food security.Front Plant Sci. 2015 May 19;6:334. doi: 10.3389/fpls.2015.00334. eCollection 2015. Front Plant Sci. 2015. PMID: 26042131 Free PMC article. Review.
-
Functional Omics Identifies Serine Hydrolases That Mobilize Storage Lipids during Rice Seed Germination.Plant Physiol. 2020 Oct;184(2):693-708. doi: 10.1104/pp.20.00268. Epub 2020 Aug 14. Plant Physiol. 2020. PMID: 32817194 Free PMC article.
-
Abscisic Acid and Abiotic Stress Tolerance in Crop Plants.Front Plant Sci. 2016 May 4;7:571. doi: 10.3389/fpls.2016.00571. eCollection 2016. Front Plant Sci. 2016. PMID: 27200044 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

