Clinical outcome measures for trials in Duchenne muscular dystrophy: report from International Working Group meetings
- PMID: 22639722
- PMCID: PMC3357954
- DOI: 10.4155/cli.11.113
Clinical outcome measures for trials in Duchenne muscular dystrophy: report from International Working Group meetings
Abstract
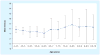
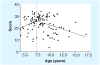
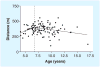
In June 2010, 25 representatives from Europe and the US met in Washington, DC, USA, to discuss clinical outcome measures in Duchenne muscular dystrophy (DMD) in the context of clinical trial design and analysis. The workshop was organized in response to a September 2009 European Medicines Agency meeting where a clear directive was given that an international consensus needs to be developed that provides a foundation for age-appropriate clinical outcome measures for use in clinical trials of emerging therapeutics for DMD. Data were presented from eight multicenter longitudinal datasets, representing nearly 1900 patients over a 20-year time period. This experience confirmed the feasibility of repeated evaluations performed at multiple sites and addressed several core issues in drug development for DMD, such as the 'new' natural history in the steroidera, reliability and sensitivity of specific outcome measures, as well as disease staging and patient selection. These data form a valuable asset for academic investigators, pharmaceutical sponsors and regulatory agencies involved in DMD therapeutics. The group remains committed working together on a number of collaborative goals to support the therapeutics development effort in this orphan disease and to make these data available to stakeholders working in the field.
Figures








References
-
- Muntoni F on behalf of the meeting Steering Committee and the TREAT-NMD Network. The development of antisense oligonucleotide therapies for Duchenne muscular dystrophy: report on a TREAT-NMD workshop hosted by the European Medicines Agency (EMA), on September 25th 2009. Neuromuscul Disord. 2010;20(5):355–362. - PubMed
-
- Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ. Clinical trial in Duchenne dystrophy. I The design of the protocol. Muscle Nerve. 1981;4(3):186–197. - PubMed
-
- Escolar DM, Henricson EK, Mayhew A, et al. Clinical evaluator reliability for quantitative and manual muscle testing measures of strength in children. Muscle Nerve. 2001;24(6):787–793. - PubMed
-
- Vignos PJJ, Spencer GEJ, Archibald KC. Management of progressive muscular dystrophy of childhood. JAMA. 1963;184:89–96. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
