Absence of amyloid β oligomers at the postsynapse and regulated synaptic Zn2+ in cognitively intact aged individuals with Alzheimer's disease neuropathology
- PMID: 22640423
- PMCID: PMC3403985
- DOI: 10.1186/1750-1326-7-23
Absence of amyloid β oligomers at the postsynapse and regulated synaptic Zn2+ in cognitively intact aged individuals with Alzheimer's disease neuropathology
Abstract
Background: Early cognitive impairment in Alzheimer Disease (AD) is thought to result from the dysfunctional effect of amyloid beta (Aβ) oligomers targeting the synapses. Some individuals, however, escape cognitive decline despite the presence of the neuropathologic features of AD (Aβ plaques and neurofibrillary tangles). We term this group Non-Demented with AD Neuropathology or NDAN. The present study illustrates one putative resistance mechanism involved in NDAN cases which may suggest targets for the effective treatment of AD.
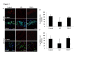
Results: Here we describe the localization of Aβ oligomers at the postsynapse in hippocampi from AD cases. Notably, however, we also found that while present in soluble fractions, Aβ oligomers are absent from hippocampal postsynapses in NDAN cases. In addition, levels of phosphorylated (active) CREB, a transcription factor important for synaptic plasticity, are normal in NDAN individuals, suggesting that their synapses are functionally intact. Analysis of Zn2+ showed that levels were increased in both soluble fractions and synaptic vesicles in AD hippocampi, paralleled by a decrease of expression of the synaptic vesicle Zn2+ transporter, ZnT3. Conversely, in NDAN individuals, levels of Zn2+ in soluble fractions were significantly lower than in AD, whereas in synaptic vesicles the levels of Zn2+ were similar to AD, but accompanied by preserved expression of the ZnT3.
Conclusions: Taken together, these data illustrate that despite substantial AD neuropathology, Aβ oligomers, and increased synaptic vesicle Zn2+, susceptible brain tissue in these aged NDAN individuals features, as compared to symptomatic AD subjects, significantly lower total Zn2+ levels and no association of Aβ oligomers with the postsynapse, which collectively may promote the maintenance of intact cognitive function.
Figures



References
-
- Alzheimer’s A. Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2010;2010:6. - PubMed
-
- Hardy J, Allsop D. Amyloid deposition as the central event in the etiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12:383–388. - PubMed
-
- Kramer PL, Xu H, Woltjer RL, Westaway SK, Clark D, Erten-Lyons D, Kaye JA, Welsh-Bohmer KA, Troncoso JC, Markesbery WR. et al.Alzheimer disease pathology in cognitively healthy elderly: A genome-wide study. Neurobiol Aging. 2011;32:2113–2122. doi: 10.1016/j.neurobiolaging.2010.01.010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

