Nef functions in BLT mice to enhance HIV-1 replication and deplete CD4+CD8+ thymocytes
- PMID: 22640559
- PMCID: PMC3403983
- DOI: 10.1186/1742-4690-9-44
Nef functions in BLT mice to enhance HIV-1 replication and deplete CD4+CD8+ thymocytes
Abstract
Background: The outcome of untreated HIV-1 infection is progression to AIDS and death in nearly all cases. Some important exceptions are the small number of patients infected with HIV-1 deleted for the accessory gene, nef. With these infections, disease progression is entirely suppressed or greatly delayed. Whether Nef is critical for high levels of replication or is directly cytotoxic remains controversial. The major problem in determining the role of Nef in HIV/AIDS has been the lack of tractable in vivo models where Nef's complex pathogenic phenotype can be recapitulated.
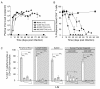
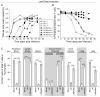
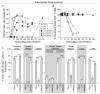
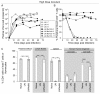
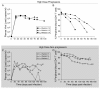
Results: Intravenous inoculation (3000 to 600,000 TCIU) of BLT humanized mice with HIV-1LAI reproducibly establishes a systemic infection. HIV-1LAI (LAI) replicates to high levels (peak viral load in blood 8,200,000 ± 1,800,000 copies of viral RNA/ml, range 3,600,000 to 20,400,000; n = 9) and exhaustively depletes CD4+ T cells in blood and tissues. CD4+CD8+ thymocytes were also efficiently depleted but CD4+CD8- thymocytes were partially resistant to cell killing by LAI. Infection with a nef-deleted LAI (LAINefdd) gave lower peak viral loads (1,220,000 ± 330,000, range 27,000 to 4,240,000; n = 17). For fourteen of seventeen LAINefdd-infected mice, there was little to no loss of either CD4+ T cells or thymocytes. Both LAI- and LAINefdd-infected mice had about 8% of total peripheral blood CD8+ T cells that were CD38+HLA-DR+ compared <1% for uninfected mice. Three exceptional LAINefdd-infected mice that lost CD4+ T cells received 600,000 TCIU. All three exhibited peak viral loads over 3,000,000 copies of LAINefdd RNA/ml. Over an extended time course, substantial systemic CD4+ T cell loss was observed for the three mice, but there was no loss of CD4+CD8+ or CD4+CD8- thymocytes.
Conclusion: We conclude Nef is necessary for elevated viral replication and as a result indirectly contributes to CD4+ T cell killing. Further, Nef was not necessary for the activation of peripheral blood CD8+ T cells following infection. However, CD4+CD8+ thymocyte killing was dependent on Nef even in cases of elevated LAINefdd replication and T cell loss. This depletion of thymic T cell precursors may be a significant factor in the elevated pathogenicity of CXCR4 trophic HIV-1.
Figures


References
-
- Okulicz JF, Marconi VC, Landrum ML, Wegner S, Weintrob A, Ganesan A, Hale B, Crum-Cianflone N, Delmar J, Barthel V. et al.Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J Infect Dis. 2009;200:1714–1723. doi: 10.1086/646609. - DOI - PubMed
-
- Churchill MJ, Rhodes DI, Learmont JC, Sullivan JS, Wesselingh SL, Cooke IR, Deacon NJ, Gorry PR. Longitudinal analysis of human immunodeficiency virus type 1 nef/long terminal repeat sequences in a cohort of long-term survivors infected from a single source. J Virol. 2006;80:1047–1052. doi: 10.1128/JVI.80.2.1047-1052.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

