The favorable impact of PIK3CA mutations on survival: an analysis of 2587 patients with breast cancer
- PMID: 22640628
- PMCID: PMC3777497
- DOI: 10.5732/cjc.012.10032
The favorable impact of PIK3CA mutations on survival: an analysis of 2587 patients with breast cancer
Abstract
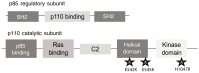
The phosphatidylinositol-3 kinase(PI3K) pathway regulates a number of cellular processes, including cell survival, cell growth, and cell cycle progression. Consequently, this pathway is commonly deregulated in cancer. In particular, mutations in the gene PIK3CA that encodes the p110α catalytic subunit of the PI3K enzymes result in cell proliferation and resistance to apoptosis in vitro and induce breast tumors in transgenic mice. These data underscore the role of this pathway during oncogenesis. Thus, an ongoing, large-scale effort is underway to develop clinically active drugs that target elements of the PI3K pathway. However, conflicting data suggest that gain-of-function PIK3CA mutations may be associated with either a favorable or a poor clinical outcome, compared with the wild-type PIK3CA gene. In the current study, we performed a systematic review of breast cancer clinical studies. Upon evaluation of 2587 breast cancer cases from 12 independent studies, we showed that patients with tumors harboring a PIK3CA mutation have a better clinical outcome than those with a wild-type PIK3CA gene. Importantly, this improved prognosis may pertain only to patients with mutations in the kinase domain of p110α and to postmenopausal women with estrogen receptor-positive breast cancer. We propose three potential explanations for this paradoxical observation. First, PIK3CA mutations may interfere with the metastasis process or may induce senescence, which results in a better outcome for patients with mutated tumors. Secondly, we speculate that PIK3CA mutations may increase early tumor diagnosis by modification of the actin cytoskeleton in tumor cells. Lastly, we propose that PIK3CA mutations may be a favorable predictive factor for response to hormonal therapy, giving a therapeutic advantage to these patients. Ultimately, an improved understanding of the clinical impact of PIK3CA mutations is critical for the development of optimally personalized therapeutics against breast cancer and other solid tumors. This effort will be important to prevent or explain therapeutic failures and select patients who are most likely to respond to new therapies that inhibit the PI3K pathway.
Figures

References
-
- Kang S, Bader AG, Zhao L, et al. et al. Mutated PI 3-kinases: cancer targets on a silver platter. Cell Cycle. 2005;4:578–581. - PubMed
-
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. - PubMed
-
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. - PubMed
-
- Katso R, Okkenhaug K, Ahmadi K, et al. et al. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
