Mechanism of the sex difference in neuronal ischemic cell death
- PMID: 22641086
- PMCID: PMC3402645
- DOI: 10.1016/j.neuroscience.2012.05.048
Mechanism of the sex difference in neuronal ischemic cell death
Abstract
Background: Stroke risk and outcome are different in men and women. We hypothesized that this is partly due to an inherent difference in susceptibility to ischemia between neurons from male vs. female brains. We tested whether neurons from male rodents are more susceptible to in-vitro ischemia than cells from females, and if this is related to increased expression of soluble epoxide hydrolase (sEH). sEH contributes to neuronal cell death by inactivating neuroprotective epoxyeicosatrienoic acids (EETs).
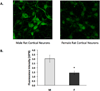
Methods: Rodent cortical neurons were cultured, and exposed to oxygen-glucose deprivation (OGD); then cell death was measured. EETs levels were determined by LC-MS/MS. Expression of sEH-encoding ephx2 was determined by qRT-PCR. Western blotting, immunocytochemistry, and hydrolase activity assay assessed protein expression and activity.
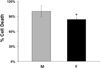
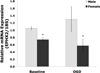
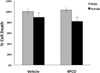
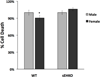
Results: Cell death after OGD was higher in neurons from males vs. females, which correlated with higher ephx2 mRNA and stronger sEH immunoreactivity. However, EETs levels were similar in both sexes and pharmacological inhibition of the hydrolase domain of sEH did not abolish the sex difference in cell death. Genetic knockout of sEH in mice abolished the sex difference observed in neurons isolated from these mice after OGD.
Conclusions: Cultured cortical neurons from females are more resistant to ischemia than neurons from males. Neurons from females have less sEH activity compared to neurons from males at baseline, although sEH levels were not measured after OGD. While pharmacological inhibition of the hydrolase domain of sEH does not affect cell death, knockout of the gene encoding sEH eradicates the sex difference seen in wild-type neurons, suggesting a role for further study of the lesser-known phosphatase domain of sEH and its role in sexual dimorphism in neuronal sensitivity to ischemia.
Copyright © 2012 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures



References
-
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998 Jan;29(1):159–165. discussion 166. - PubMed
-
- Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000 Jan;31(1):161–168. - PubMed
-
- Bernstrom K, Kayganich K, Murphy RC, Fitzpatrick FA. Incorporation and distribution of epoxyeicosatrienoic acids into cellular phospholipids. The Journal of biological chemistry. 1992 Feb 25;267(6):3686–3690. - PubMed
-
- Du Lina, Bayir Hülya, Lai Yichen, Zhang Xiaopeng, Kochanek Patrick M, Watkins Simon C, Graham Steven H, Clark Robert S B. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. The Journal of biological chemistry. 2004 Sep 10;279(37):38563–38570. - PubMed
-
- Golomb MR, Fullerton HJ, Nowak-Gottl U, deVeber G for the International Pediatric Stroke Study Group. Male Predominance in Childhood Ischemic Stroke: Findings From the International Pediatric Stroke Study. Stroke. 2008 Dec 29;40(1):52–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

