Responder analysis of the effects of denosumab on bone mineral density in men receiving androgen deprivation therapy for prostate cancer
- PMID: 22641239
- PMCID: PMC3671885
- DOI: 10.1038/pcan.2012.18
Responder analysis of the effects of denosumab on bone mineral density in men receiving androgen deprivation therapy for prostate cancer
Abstract
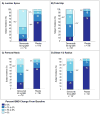
Background: Denosumab, a fully human monoclonal antibody against RANK ligand, increased bone mineral density (BMD) and reduced fracture risk vs placebo in a phase 3 trial in men with prostate cancer on androgen deprivation therapy (ADT). The present analysis of this study evaluated BMD changes after 36 months in responder subgroups and in individual patients for three key skeletal sites (lumbar spine (LS), femoral neck (FN) and total hip (TH)) and the distal radius.
Methods: Men with nonmetastatic prostate cancer receiving ADT were treated with subcutaneous denosumab 60 mg (n=734) or placebo (n=734) every 6 months for up to 36 months in a phase 3, randomized, double-blind study. Patients were instructed to take supplemental calcium and vitamin D. For this BMD responder analysis, the primary outcome measure was the percentage change in BMD from baseline to month 36 at the LS, FN and TH as measured by dual-energy X-ray absorptiometry. BMD at the distal 1/3 radius at 36 months was measured in a substudy of 309 patients.
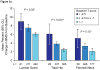
Results: At 36 months, significantly more patients in the denosumab arm had increases of >3% BMD from baseline at each site studied compared with placebo (LS, 78 vs 17%; FN, 48 vs 13%; TH, 48 vs 6%; distal 1/3 radius, 40 vs 7% (P<0.0001 for all)). BMD loss at the LS, FN and TH occurred in 1% of denosumab-treated patients vs 42% of placebo patients, and BMD gain at all three sites occurred in 69% of denosumab patients vs 8% of placebo patients. Lower baseline BMD was associated with higher-magnitude BMD responses to denosumab at the LS, FN and TH.
Conclusions: In men with prostate cancer receiving ADT, significantly higher BMD response rates were observed with denosumab vs placebo. Patients with lower baseline T-scores benefited the most from denosumab treatment.
Conflict of interest statement
Fred Saad: Sanofi-Aventis, Novartis, Amgen grant support; Sanofi-Aventis, Novartis, Amgen consulting and honoraria.
Matthew R Smith: Amgen, GTx, Novartis consulting; Amgen, GTx honoraria
Teuvo LJ Tammela: Orion Pharma, AstraZeneca consulting; AstraZeneca, Astellas honoraria
Jiri Heracek: Amgen consulting.
Paul Sieber: Amgen, GTx consulting.
Chunlei Ke: Amgen employee, stock ownership
Benjamin Leder: Amgen, New England Research Institutes consulting; Merck, Novartis honoraria.
Roger Dansey: Amgen employee, stock ownership
Carsten Goessl: Amgen employee, stock ownership
Figures



References
-
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in europe in 2008. European Journal of Cancer. 2010;46:765–81. - PubMed
-
- Brawley OW. Prostate carcinoma incidence and patient mortality: The effects of screening and early detection. Cancer. 1997;80:1857–63. - PubMed
-
- La Vecchia C, Bosetti C, Lucchini F, Bertuccio P, Negri E, Boyle P, et al. Cancer mortality in europe, 2000–2004, and an overview of trends since 1975. Annals of Oncology. 2009 - PubMed
-
- Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, et al. Eau guidelines on prostate cancer. Eur Urol. 2008;53:68–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

