Potent and broad neutralization of HIV-1 by a llama antibody elicited by immunization
- PMID: 22641382
- PMCID: PMC3371729
- DOI: 10.1084/jem.20112655
Potent and broad neutralization of HIV-1 by a llama antibody elicited by immunization
Abstract
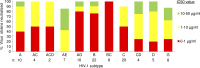
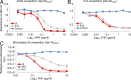
Llamas (Lama glama) naturally produce heavy chain-only antibodies (Abs) in addition to conventional Abs. The variable regions (VHH) in these heavy chain-only Abs demonstrate comparable affinity and specificity for antigens to conventional immunoglobulins despite their much smaller size. To date, immunizations in humans and animal models have yielded only Abs with limited ability to neutralize HIV-1. In this study, a VHH phagemid library generated from a llama that was multiply immunized with recombinant trimeric HIV-1 envelope proteins (Envs) was screened directly for HIV-1 neutralization. One VHH, L8CJ3 (J3), neutralized 96 of 100 tested HIV-1 strains, encompassing subtypes A, B, C, D, BC, AE, AG, AC, ACD, CD, and G. J3 also potently neutralized chimeric simian-HIV strains with HIV subtypes B and C Env. The sequence of J3 is highly divergent from previous anti-HIV-1 VHH and its own germline sequence. J3 achieves broad and potent neutralization of HIV-1 via interaction with the CD4-binding site of HIV-1 Env. This study may represent a new benchmark for immunogens to be included in B cell-based vaccines and supports the development of VHH as anti-HIV-1 microbicides.
Figures





References
-
- Abdool Karim Q., Abdool Karim S.S., Frohlich J.A., Grobler A.C., Baxter C., Mansoor L.E., Kharsany A.B., Sibeko S., Mlisana K.P., Omar Z., et al. ; CAPRISA 004 Trial Group 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 329:1168–1174 10.1126/science.1193748 - DOI - PMC - PubMed
-
- Baba T.W., Liska V., Hofmann-Lehmann R., Vlasak J., Xu W., Ayehunie S., Cavacini L.A., Posner M.R., Katinger H., Stiegler G., et al. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200–206 10.1038/72309 - DOI - PubMed
-
- Brinckmann S., da Costa K., van Gils M.J., Hallengärd D., Klein K., Madeira L., Mainetti L., Palma P., Raue K., Reinhart D., et al. 2011. Rational design of HIV vaccines and microbicides: report of the EUROPRISE network annual conference 2010. J. Transl. Med. 9:40 10.1186/1479-5876-9-40 - DOI - PMC - PubMed
-
- Buchacher A., Predl R., Strutzenberger K., Steinfellner W., Trkola A., Purtscher M., Gruber G., Tauer C., Steindl F., Jungbauer A., et al. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retroviruses. 10:359–369 10.1089/aid.1994.10.359 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

