Toxofilin upregulates the host cortical actin cytoskeleton dynamics, facilitating Toxoplasma invasion
- PMID: 22641695
- PMCID: PMC3516439
- DOI: 10.1242/jcs.103648
Toxofilin upregulates the host cortical actin cytoskeleton dynamics, facilitating Toxoplasma invasion
Abstract
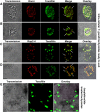
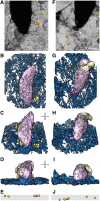
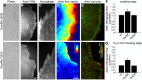
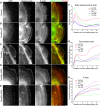
Toxoplasma gondii, a human pathogen and a model apicomplexan parasite, actively and rapidly invades host cells. To initiate invasion, the parasite induces the formation of a parasite-cell junction, and progressively propels itself through the junction, inside a newly formed vacuole that encloses the entering parasite. Little is known about how a parasite that is a few microns in diameter overcomes the host cell cortical actin barrier to achieve the remarkably rapid process of internalization (less than a few seconds). Using correlative light and electron microscopy in conjunction with electron tomography and three-dimensional image analysis we identified that toxofilin, an actin-binding protein, secreted by invading parasites correlates with localized sites of disassembly of the host cell actin meshwork. Moreover, quantitative fluorescence speckle microscopy of cells expressing toxofilin showed that toxofilin regulates actin filament disassembly and turnover. Furthermore, Toxoplasma tachyzoites lacking toxofilin, were found to be impaired in cortical actin disassembly and exhibited delayed invasion kinetics. We propose that toxofilin locally upregulates actin turnover thus increasing depolymerization events at the site of entry that in turn loosens the local host cell actin meshwork, facilitating parasite internalization and vacuole folding.
Figures




References
-
- Bradley P. J., Ward C., Cheng S. J., Alexander D. L., Coller S., Coombs G. H., Dunn J. D., Ferguson D. J., Sanderson S. J., Wastling J. M., et al. (2005). Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J. Biol. Chem. 280, 34245–34258 10.1074/jbc.M504158200 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

