The photovoltage of rods and cones in the dark-adapted mouse retina
- PMID: 22641773
- PMCID: PMC3476636
- DOI: 10.1113/jphysiol.2011.226878
The photovoltage of rods and cones in the dark-adapted mouse retina
Abstract
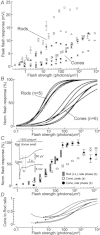
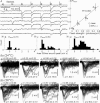
Research on photoreceptors has led to important insights into how light signals are detected and processed in the outer retina. Most information about photoreceptor function, however, comes from lower vertebrates. The large majority of mammalian studies are based on suction pipette recordings of outer segment currents, a technique that doesn't allow examination of phenomena occurring downstream of phototransduction. Only a small number of whole-cell recordings have been made, mainly in the macaque. Due to the growing importance of the mouse in vision research, we have optimized a retinal slice preparation that allows the reliable collection of perforated-patch recordings from light responding rods and cones. Unexpectedly, the frequency of cone recordings was much higher than their numeric proportion of ∼3%. This allowed us to obtain direct functional evidence suggestive of rod–cone coupling in the mouse. Moreover, rods had considerably larger single photon responses than previously published for mammals (3.44 mV, SD 1.37, n = 19 at 24°C; 2.46 mV, SD 1.08, n = 10 at 36°C), and a relatively high signal/noise ratio (6.4, SD 1.8 at 24°C; 6.8, SD 2.8 at 36°C). Both findings imply a more favourable transmission at the rod–rod bipolar cell synapse. Accordingly, relatively few photoisomerizations were sufficient to elicit a half-maximal response (6.7, SD 2.7, n = 5 at 24°C; 10.6, SD 1.7, n = 3 at 36°C), leading to a narrow linear response range. Our study demonstrates new features of mammalian photoreceptors and opens the way for further investigations into photoreceptor function using retinas from mutant mouse models.
Figures



Comment in
-
New light on photon detection.J Physiol. 2012 Aug 15;590(16):3641-2. doi: 10.1113/jphysiol.2012.237040. J Physiol. 2012. PMID: 22904363 Free PMC article. No abstract available.
References
-
- Agresti A, Coull BA. Approximate is better than ‘exact’ for interval estimation of binomial proportions. Amer Statist. 1998;52:119–126.
-
- Akaike N, Harata N. Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms. Jpn J Physiol. 1994;44:433–473. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

