Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease
- PMID: 22642194
- PMCID: PMC3367802
- DOI: 10.1007/978-3-0348-0364-9_7
Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease
Abstract
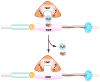
Matrix metalloproteinases (MMPs) are proteolytic enzymes that degrade various components of the extracellular matrix (ECM). MMPs could also regulate the activity of several non-ECM bioactive substrates and consequently affect different cellular functions. Members of the MMPs family include collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs, and others. Pro-MMPs are cleaved into active MMPs, which in turn act on various substrates in the ECM and on the cell surface. MMPs play an important role in the regulation of numerous physiological processes including vascular remodeling and angiogenesis. MMPs may also be involved in vascular diseases such as hypertension, atherosclerosis, aortic aneurysm, and varicose veins. MMPs also play a role in the hemodynamic and vascular changes associated with pregnancy and preeclampsia. The role of MMPs is commonly assessed by measuring their gene expression, protein amount, and proteolytic activity using gel zymography. Because there are no specific activators of MMPs, MMP inhibitors are often used to investigate the role of MMPs in different physiologic processes and in the pathogenesis of specific diseases. MMP inhibitors include endogenous tissue inhibitors (TIMPs) and pharmacological inhibitors such as zinc chelators, doxycycline, and marimastat. MMP inhibitors have been evaluated as diagnostic and therapeutic tools in cancer, autoimmune disease, and cardiovascular disease. Although several MMP inhibitors have been synthesized and tested both experimentally and clinically, only one MMP inhibitor, i.e., doxycycline, is currently approved by the Food and Drug Administration. This is mainly due to the undesirable side effects of MMP inhibitors especially on the musculoskeletal system. While most experimental and clinical trials of MMP inhibitors have not demonstrated significant benefits, some trials still showed promising results. With the advent of new genetic and pharmacological tools, disease-specific MMP inhibitors with fewer undesirable effects are being developed and could be useful in the management of vascular disease.
Figures
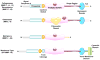
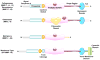
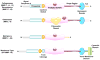

References
-
- Aguilera CM, George SJ, Johnson JL, Newby AC. Relationship between type IV collagen degradation, metalloproteinase activity and smooth muscle cell migration and proliferation in cultured human saphenous vein. Cardiovasc Res. 2003;58:679–688. - PubMed
-
- Ahokas K, Lohi J, Illman SA, Llano E, Elomaa O, Impola U, Karjalainen-Lindsberg ML, Saarialho-Kere U. Matrix metalloproteinase-21 is expressed epithelially during development and in cancer and is up-regulated by transforming growth factor-beta1 in keratinocytes. Lab Invest. 2003;83:1887–1899. - PubMed
-
- Aikawa M, Rabkin E, Voglic SJ, Shing H, Nagai R, Schoen FJ, Libby P. Lipid lowering promotes accumulation of mature smooth muscle cells expressing smooth muscle myosin heavy chain isoforms in rabbit atheroma. Circ Res. 1998;83:1015–1026. - PubMed
-
- Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995;270:5872–5876. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
