Selective estrogen receptor modulator (SERM) lasofoxifene forms reactive quinones similar to estradiol
- PMID: 22642258
- PMCID: PMC3398215
- DOI: 10.1021/tx300142h
Selective estrogen receptor modulator (SERM) lasofoxifene forms reactive quinones similar to estradiol
Abstract
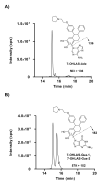
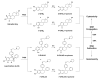
The bioactivation of both endogenous and equine estrogens to electrophilic quinoid metabolites has been postulated as a contributing factor in carcinogenic initiation and/or promotion in hormone sensitive tissues. Bearing structural resemblance to estrogens, extensive studies have shown that many selective estrogen receptor modulators (SERMs) are subject to similar bioactivation pathways. Lasofoxifene (LAS), a third generation SERM which has completed phase III clinical trials for the prevention and treatment of osteoporosis, is currently approved in the European Union for this indication. Previously, Prakash et al. (Drug Metab. Dispos. (2008) 36, 1218-1226) reported that similar to estradiol, two catechol regioisomers of LAS are formed as primary oxidative metabolites, accounting for roughly half of the total LAS metabolism. However, the potential for further oxidation of these catechols to electrophilic o-quinones has not been reported. In the present study, LAS was synthesized and its oxidative metabolism investigated in vitro under various conditions. Incubation of LAS with tyrosinase, human liver microsomes, or rat liver microsomes in the presence of GSH as a trapping reagent resulted in the formation of two mono-GSH and two di-GSH catechol conjugates which were characterized by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Similar conjugates were also detected in incubations with P450 3A4, P450 2D6, and P450 1B1 supersomes. Interestingly, these conjugates were also detected as major metabolites when compared to competing detoxification pathways such as glucuronidation and methylation. The 7-hydroxylasofoxifene (7-OHLAS) catechol regioisomer was also synthesized and oxidized either chemically or enzymatically to an o-quinone that was shown to form depurinating adducts with DNA. Collectively, these data show that analogous to estrogens, LAS is oxidized to catechols and o-quinones which could potentially contribute to in vivo toxicity for this SERM.
Figures






References
-
- Cho CH, Nuttall ME. Therapeutic potential of oestrogen receptor ligands in development for osteoporosis. Expert Opin. Emerg. Drugs. 2001;6:137–154. - PubMed
-
- Shelly W, Draper MW, Krishnan V, Wong M, Jaffe RB. Selective estrogen receptor modulators: an update on recent clinical findings. Obstet. Gynecol. Surv. 2008;63:163–181. - PubMed
-
- Peterson GM, Naunton M, Tichelaar LK, Gennari L. Lasofoxifene: selective estrogen receptor modulator for the prevention and treatment of postmenopausal osteoporosis. Ann. Pharmacother. 2011;45:499–509. - PubMed
-
- Gennari L, Merlotti D, Stolakis K, Nuti R. Lasofoxifene, from the preclinical drug discovery to the treatment of postmenopausal osteoporosis. Expert Opin. Drug Discovery. 2011;6:205–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

