Field effects induce bathochromic shifts in xanthene dyes
- PMID: 22642754
- PMCID: PMC3384756
- DOI: 10.1021/ja302445w
Field effects induce bathochromic shifts in xanthene dyes
Abstract
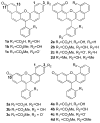
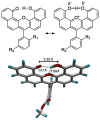
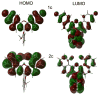
There is ongoing interest in near-infrared (NIR) absorbing and emitting dyes for a variety of biomedical and materials applications. Simple and efficient synthetic procedures enable the judicious tuning of through-space polar (field) effects as well as low barrier hydrogen bonding to modulate the HOMO-LUMO gap in xanthene dyes. This affords unique NIR-absorbing xanthene chromophores.
Conflict of interest statement
The authors declare no competing financial interest
Figures



Similar articles
-
Silicon-substituted Xanthene Dyes and Their Unique Photophysical Properties for Fluorescent Probes.Chem Asian J. 2017 Jul 4;12(13):1435-1446. doi: 10.1002/asia.201700385. Epub 2017 Jun 5. Chem Asian J. 2017. PMID: 28452155 Review.
-
Development of unique xanthene-cyanine fused near-infrared fluorescent fluorophores with superior chemical stability for biological fluorescence imaging.Chemistry. 2015 Jan 7;21(2):733-45. doi: 10.1002/chem.201404718. Epub 2014 Nov 11. Chemistry. 2015. PMID: 25388080
-
Xanthene-based NIR organic phototheranostics agents: design strategies and biomedical applications.J Mater Chem B. 2025 Feb 26;13(9):2952-2977. doi: 10.1039/d4tb02480j. J Mater Chem B. 2025. PMID: 39898613 Review.
-
Thienylpiperidine Donor NIR Xanthene-Based Dye for Photoacoustic Imaging.Org Lett. 2021 Oct 1;23(19):7640-7644. doi: 10.1021/acs.orglett.1c02862. Epub 2021 Sep 22. Org Lett. 2021. PMID: 34550707
-
Altering Fundamental Trends in the Emission of Xanthene Dyes.J Org Chem. 2019 Mar 1;84(5):2585-2595. doi: 10.1021/acs.joc.8b03030. Epub 2019 Feb 15. J Org Chem. 2019. PMID: 30719911
Cited by
-
Near-infrared fluorescein dyes containing a tricoordinate boron atom.Chem Sci. 2019 Jul 3;10(33):7816-7821. doi: 10.1039/c9sc02314c. eCollection 2019 Sep 7. Chem Sci. 2019. PMID: 31588332 Free PMC article.
-
The pursuit of xanthenoid fluorophores with near-infrared-II emission for in vivo applications.Anal Bioanal Chem. 2023 Jul;415(18):3789-3797. doi: 10.1007/s00216-022-04463-z. Epub 2022 Nov 29. Anal Bioanal Chem. 2023. PMID: 36445453 Review.
-
Designing Red-Shifted Molecular Emitters Based on the Annulated Locked GFP Chromophore Derivatives.Int J Mol Sci. 2021 Dec 20;22(24):13645. doi: 10.3390/ijms222413645. Int J Mol Sci. 2021. PMID: 34948442 Free PMC article.
-
Metal-Free Nitrogen-Containing Polyheterocyclic Near-Infrared (NIR) Absorption Dyes: Synthesis, Absorption Properties, and Theoretical Calculation of Substituted 5-Methylisoindolo[2,1-a]quinolines.ACS Omega. 2019 Mar 8;4(3):5064-5075. doi: 10.1021/acsomega.9b00315. eCollection 2019 Mar 31. ACS Omega. 2019. PMID: 31459684 Free PMC article.
-
Voltage Imaging with a NIR-Absorbing Phosphine Oxide Rhodamine Voltage Reporter.J Am Chem Soc. 2021 Feb 10;143(5):2304-2314. doi: 10.1021/jacs.0c11382. Epub 2021 Jan 27. J Am Chem Soc. 2021. PMID: 33501825 Free PMC article.
References
-
- Alford R, Simpson HM, Duberman J, Hill GC, Ogawa M, Regino C, Kobayashi H, Choyke PL. Mol Imaging. 2009;8:341. - PubMed
-
- Philip R, Penzkofer A, Baumler W, Szeimies RM, Abels C. J Photochem Photobiol A-Chem. 1996;96:137.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

