Exacerbated metastatic disease in a mouse mammary tumor model following latent gammaherpesvirus infection
- PMID: 22642913
- PMCID: PMC3565933
- DOI: 10.1186/1750-9378-7-11
Exacerbated metastatic disease in a mouse mammary tumor model following latent gammaherpesvirus infection
Abstract
Background: Controversy exists as to the ability of human gammaherpesviruses to cause or exacerbate breast cancer disease in patients. The difficulty in conducting definitive human studies can be overcome by investigating developing breast cancer in a mouse model. In this study, we utilized mice latently infected with murine gammaherpesvirus 68 (HV-68) to question whether such a viral burden could exacerbate metastatic breast cancer disease using a mouse mammary tumor model.
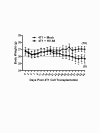
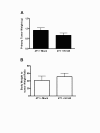
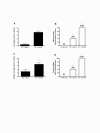
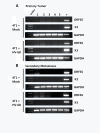
Results: Mice latently infected with HV-68 had a similar primary tumor burden, but much greater metastatic disease, when compared to mock treated mice given the transplantable tumor, 4 T1. This was true for lung lesions, as well as secondary tumor masses. Increased expression of pan-cytokeratin and VEGF-A in tumors from HV-68 infected mice was consistent with increased metastatic disease in these animals. Surprisingly, no viral particles could be cultured from tumor tissues, and the presence of viral DNA or RNA transcripts could not be detected in primary or secondary tumor tissues.
Conclusions: Latent HV-68 infection had no significant effect on the size of primary 4 T1 mammary tumors, but exacerbated the number of metastatic lung lesions and secondary tumors when compared to mock treated mice. Increased expression of the tumor marker, pan-cytokeratin, and VEGF-A in tumors of mice harboring latent virus was consistent with an exacerbated metastatic disease. Mechanisms responsible for this exacerbation are indirect, since no virus could be detected in cancerous tissues.
Figures



Similar articles
-
An expanded myeloid derived suppressor cell population does not play a role in gammaherpesvirus-exacerbated breast cancer metastases.Infect Agent Cancer. 2012 Sep 4;7(1):22. doi: 10.1186/1750-9378-7-22. Infect Agent Cancer. 2012. PMID: 22946998 Free PMC article.
-
Infection with murine gammaherpesvirus 68 exacerbates inflammatory bowel disease in IL-10-deficient mice.Inflamm Res. 2009 Dec;58(12):881-9. doi: 10.1007/s00011-009-0059-x. Epub 2009 Jun 21. Inflamm Res. 2009. PMID: 19544045
-
Latent infection by γherpesvirus stimulates profibrotic mediator release from multiple cell types.Am J Physiol Lung Cell Mol Physiol. 2011 Feb;300(2):L274-85. doi: 10.1152/ajplung.00028.2010. Epub 2010 Oct 29. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21036917 Free PMC article.
-
Establishment of cell lines latently infected with non-oncogenic murine gammaherpesvirus 76.Acta Virol. 2010;54(4):287-91. doi: 10.4149/av_2010_04_287. Acta Virol. 2010. PMID: 21175252
-
Interleukin-27 expression following infection with the murine gammaherpesvirus 68.Cytokine. 2010 Aug;51(2):184-94. doi: 10.1016/j.cyto.2010.04.015. Epub 2010 May 20. Cytokine. 2010. PMID: 20493722
Cited by
-
Prevention of Tumor Formation by Latent Gammaherpesvirus Infection.PLoS One. 2015 Dec 29;10(12):e0145678. doi: 10.1371/journal.pone.0145678. eCollection 2015. PLoS One. 2015. PMID: 26714031 Free PMC article.
-
An expanded myeloid derived suppressor cell population does not play a role in gammaherpesvirus-exacerbated breast cancer metastases.Infect Agent Cancer. 2012 Sep 4;7(1):22. doi: 10.1186/1750-9378-7-22. Infect Agent Cancer. 2012. PMID: 22946998 Free PMC article.
-
Alpha beta-crystallin expression and presentation following infection with murine gammaherpesvirus 68.Autoimmunity. 2013 Sep;46(6):399-408. doi: 10.3109/08916934.2013.785535. Epub 2013 Apr 16. Autoimmunity. 2013. PMID: 23586607 Free PMC article.
References
-
- Glaser SL, Hsu JL, Gulley ML. Epstein-Barr virus and breast cancer: state of the evidence for viral carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2004;13(5):688–697. - PubMed
-
- Joshi D, Buehring GC. Are viruses associated with human breast cancer? Breast Cancer Res Treat: Scrutinizing the molecular evidence; 2012. - PubMed
LinkOut - more resources
Full Text Sources

