Targeting the hemoglobin scavenger receptor CD163 in macrophages highly increases the anti-inflammatory potency of dexamethasone
- PMID: 22643864
- PMCID: PMC3412497
- DOI: 10.1038/mt.2012.103
Targeting the hemoglobin scavenger receptor CD163 in macrophages highly increases the anti-inflammatory potency of dexamethasone
Abstract
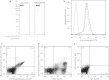
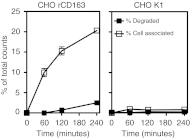
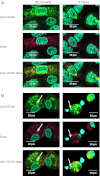
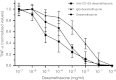
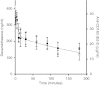
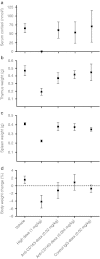
Synthetic glucocorticoids are potent anti-inflammatory drugs but serious side effects such as bone mobilization, muscle mass loss, immunosuppression, and metabolic alterations make glucocorticoid therapy a difficult balance. The therapeutic anti-inflammatory effect of glucocorticoids relies largely on the suppressed release of tumor-necrosis factor-α and other cytokines by macrophages at the sites of inflammation. We have now developed a new biodegradable anti-CD163 antibody-drug conjugate that specifically targets the glucocorticoid, dexamethasone to the hemoglobin scavenger receptor CD163 in macrophages. The conjugate, that in average contains four dexamethasone molecules per antibody, exhibits retained high functional affinity for CD163. In vitro studies in rat macrophages and in vivo studies of Lewis rats showed a strong anti-inflammatory effect of the conjugate measured as reduced lipopolysaccharide-induced secretion of tumor-necrosis factor-α. The in vivo potency of conjugated dexamethasone was about 50-fold that of nonconjugated dexamethasone. In contrast to a strong systemic effect of nonconjugated dexamethasone, the equipotent dose of the conjugate had no such effect, measured as thymus lymphocytes apoptosis, body weight loss, and suppression of endogenous cortisol levels. In conclusion, the study shows antibody-drug conjugates as a future approach in anti-inflammatory macrophage-directed therapy. Furthermore, the data demonstrate CD163 as an excellent macrophage target for anti-inflammatory drug delivery.
Figures


References
-
- Rhen T., and, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. - PubMed
-
- Clark IA. How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev. 2007;18:335–343. - PubMed
-
- Saklatvala J, Dean J., and, Clark A. Control of the expression of inflammatory response genes. Biochem Soc Symp. 2003;70:95–106. - PubMed
-
- Dayer JM. The process of identifying and understanding cytokines: from basic studies to treating rheumatic diseases. Best Pract Res Clin Rheumatol. 2004;18:31–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

