Recent advances in azaborine chemistry
- PMID: 22644658
- PMCID: PMC3698317
- DOI: 10.1002/anie.201200063
Recent advances in azaborine chemistry
Abstract
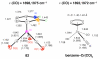
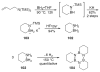
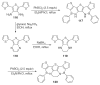
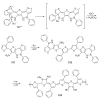
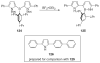
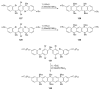
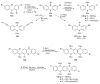
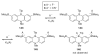
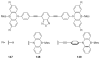
The chemistry of organoboron compounds has been primarily dominated by their use as powerful reagents in synthetic organic chemistry. Recently, the incorporation of boron as part of a functional target structure has emerged as a useful way to generate diversity in organic compounds. A commonly applied strategy is the replacement of a CC unit with its isoelectronic BN unit. In particular, the BN/CC isosterism of the ubiquitous arene motif has undergone a renaissance in the past decade. The parent molecule of the 1,2-dihydro-1,2-azaborine family has now been isolated. New mono- and polycyclic B,N heterocycles have been synthesized for potential use in biomedical and materials science applications. This review is a tribute to Dewar's first synthesis of a monocyclic 1,2-dihydro-1,2-azaborine 50 years ago and discusses recent advances in the synthesis and characterization of heterocycles that contain carbon, boron, and nitrogen.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

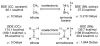
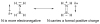
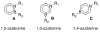



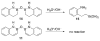
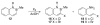

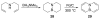
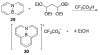
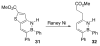
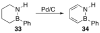

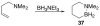

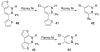




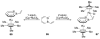
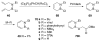
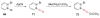

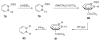


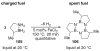
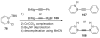

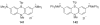
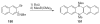

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

