MR liver imaging with Gd-EOB-DTPA: a delay time of 10 minutes is sufficient for lesion characterisation
- PMID: 22645040
- PMCID: PMC3431472
- DOI: 10.1007/s00330-012-2486-2
MR liver imaging with Gd-EOB-DTPA: a delay time of 10 minutes is sufficient for lesion characterisation
Abstract
Objectives: To assess whether, in patients with normal liver function, a hepatobiliary delay time of 10 min after Gd-EOB-DTPA injection is sufficient for lesion characterisation.
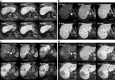
Methods: In 42 consecutive patients with suspected focal liver lesions, dynamic MRI was performed after intravenous Gd-EOB-DTPA, followed by hepatobiliary phases at 5, 10 and 20 min. The following items were assessed at each hepatobiliary phase: parenchymal enhancement, contrast agent excretion in bile ducts, lesion enhancement characteristics (hypo-, iso-, or hyperintensity, rim enhancement, central non-enhancement), and contrast- and signal-to-noise ratios, separately for hypo- and hyperintense lesions.
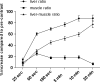
Results: Following enhancement, parenchymal signal intensity increased significantly up to 10 min (86.3%, P < 0.001), and subsequently stabilised (86.5% after 20 min, P = 0.223). Biliary contrast agent excretion was first observed in 2, 32 and 5 patients after 5, 10 and 20 min respectively. Hepatobiliary lesion enhancement characteristics observed after 5 min persisted during later hepatobiliary phases. CNR and SNR ratios increased significantly (P < 0.05) up to 10 min after enhancement without further increase at 20 min, in hypo- and hyperintense lesions.
Conclusions: If lesion characterisation is the primary reason for performing MRI, a hepatobiliary delay time of 10 min after Gd-EOB-DTPA injection is sufficient in patients with normal liver function.
Key points: • Magnetic resonance imaging is now a first line of investigation of the liver. • Optimal CNR and SNR are achieved 10 min after Gd-EOB-DTPA injection. • Typical enhancement characteristics are observed early and do not change. • Ten-minute hepatobiliary delay is sufficient for characterisation of focal liver lesions.
Figures


References
-
- Vogl TJ, Pegios W, McMahon C, et al. Gadobenate dimeglumine—a new contrast agent for MR imaging: preliminary evaluation in healthy volunteers. AJR Am J Roentgenol. 1992;158:887–892. - PubMed
-
- Reimer P, Rummeny EJ, Shamsi K, et al. Phase II clinical evaluation of Gd-EOB-DTPA: dose, safety aspects, and pulse sequence. Radiology. 1996;199:177–183. - PubMed
-
- Hammerstingl R, Huppertz A, Breuer J, et al. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol. 2008;18:457–467. doi: 10.1007/s00330-007-0716-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

