Mechanistic insights into regulated cargo binding by ACAP1 protein
- PMID: 22645133
- PMCID: PMC3436524
- DOI: 10.1074/jbc.M112.378810
Mechanistic insights into regulated cargo binding by ACAP1 protein
Abstract
Coat complexes sort protein cargoes into vesicular transport pathways. An emerging class of coat components has been the GTPase-activating proteins (GAPs) that act on the ADP-ribosylation factor (ARF) family of small GTPases. ACAP1 (ArfGAP with coiled-coil, ankyrin repeat, and PH domains protein 1) is an ARF6 GAP that also acts as a key component of a recently defined clathrin complex for endocytic recycling. Phosphorylation by Akt has been shown to enhance cargo binding by ACAP1 in explaining how integrin recycling is an example of regulated transport. We now shed further mechanistic insights into how this regulation is achieved at the level of cargo binding by ACAP1. We initially defined a critical sequence in the cytoplasmic domain of integrin β1 recognized by ACAP1 and showed that this sequence acts as a recycling sorting signal. We then pursued a combination of structural, modeling, and functional studies, which suggest that phosphorylation of ACAP1 relieves a localized mechanism of autoinhibition in regulating cargo binding. Thus, we have elucidated a key regulatory juncture that controls integrin recycling and also advanced the understanding of how regulated cargo binding can lead to regulated transport.
Figures
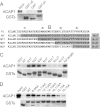
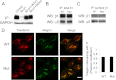
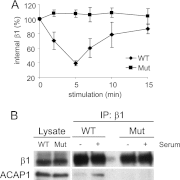

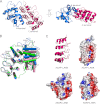

References
-
- Bonifacino J. S., Glick B. S. (2004) The mechanisms of vesicle budding and fusion. Cell 116, 153–166 - PubMed
-
- Caswell P., Norman J. (2008) Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 18, 257–263 - PubMed
-
- Pellinen T., Ivaska J. (2006) Integrin traffic. J. Cell Sci. 119, 3723–3731 - PubMed
-
- Margadant C., Monsuur H. N., Norman J. C., Sonnenberg A. (2011) Mechanisms of integrin activation and trafficking. Curr. Opin. Cell Biol. 23, 607–614 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

