Localization of Aggregatibacter actinomycetemcomitans cytolethal distending toxin subunits during intoxication of live cells
- PMID: 22645284
- PMCID: PMC3434584
- DOI: 10.1128/IAI.00385-12
Localization of Aggregatibacter actinomycetemcomitans cytolethal distending toxin subunits during intoxication of live cells
Abstract
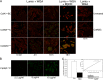
The cytolethal distending toxin (Cdt), produced by some clinically important Gram-negative bacterial species, is related to the family of AB-type toxins. Three heterologous proteins (CdtA, CdtB, and CdtC) and a genotoxin mode of action distinguish the Cdt from others in this toxin class. Crystal structures of several species-specific Cdts have provided a basis for predicting subunit interactions and functions. In addition, empirical studies have yielded significant insights into the in vivo interactions of the Cdt subunits. However, there are still critical gaps in information about the intoxication process. In this study, a novel protein tagging technology was used to localize the subunits in Chinese hamster ovary cells (CHO-K1). A tetracysteine motif was engineered in each subunit, and in subunits with mutations in predicted functional domains, to permit detection with the fluorescein arsenical hairpin binding (FlAsH) dye Lumio green. Live-cell imaging, in conjunction with confocal microscopy, was used to capture the locations of the individual subunits in cells intoxicated, under various conditions, with hybrid heterotrimers. Using this approach, we observed the following. (i) The CdtA subunit remains on the cell surface of CHO cells in association with cholesterol-containing and cholesterol-depleted membrane. (ii) The CdtB subunit is exclusively in the cytosol and, after longer exposure times, localizes to the nucleus. (iii) The CdtC subunit is present on the cell surface and, to a greater extent, in the cytosol. These observations suggest that CdtC, but not CdtA, functions as a chaperone for CdtB entry into cells.
Figures






References
-
- Akifusa S, Heywood W, Nair SP, Stenbeck G, Henderson B. 2005. Mechanism of internalization of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Microbiology 151:1395–1402 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

