Sex-lethal enables germline stem cell differentiation by down-regulating Nanos protein levels during Drosophila oogenesis
- PMID: 22645327
- PMCID: PMC3386055
- DOI: 10.1073/pnas.1120473109
Sex-lethal enables germline stem cell differentiation by down-regulating Nanos protein levels during Drosophila oogenesis
Abstract
Drosophila ovarian germ cells require Sex-lethal (Sxl) to exit from the stem cell state and to enter the differentiation pathway. Sxl encodes a female-specific RNA binding protein and in somatic cells serves as the developmental switch gene for somatic sex determination and X-chromosome dosage compensation. None of the known Sxl target genes are required for germline differentiation, leaving open the question of how Sxl promotes the transition from stem cell to committed daughter cell. We address the mechanism by which Sxl regulates this transition through the identification of nanos as one of its target genes. Previous studies have shown that Nanos protein is necessary for GSC self-renewal and is rapidly down-regulated in the daughter cells fated to differentiate in the adult ovary. We find that this dynamic expression pattern is limited to female germ cells and is under Sxl control. In the absence of Sxl, or in male germ cells, Nanos protein is continuously expressed. Furthermore, this female-specific expression pattern is dependent on the presence of canonical Sxl binding sites located in the nanos 3' untranslated region. These results, combined with the observation that nanos RNA associates with the Sxl protein in ovarian extracts and loss and gain of function studies, suggest that Sxl enables the switch from germline stem cell to committed daughter cell by posttranscriptional down-regulation of nanos expression. These findings connect sexual identity to the stem cell self-renewal/differentiation decision and highlight the importance of posttranscriptional gene regulatory networks in controlling stem cell behavior.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
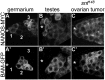

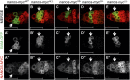
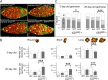

Similar articles
-
Sex, stem cells and tumors in the Drosophila ovary.Fly (Austin). 2013 Jan-Mar;7(1):3-7. doi: 10.4161/fly.22687. Epub 2012 Dec 3. Fly (Austin). 2013. PMID: 23208193 Free PMC article.
-
Maintenance of Drosophila germline stem cell sexual identity in oogenesis and tumorigenesis.Development. 2015 Mar 15;142(6):1073-82. doi: 10.1242/dev.116590. Development. 2015. PMID: 25758221 Free PMC article.
-
Sex-lethal is a target of Bruno-mediated translational repression in promoting the differentiation of stem cell progeny during Drosophila oogenesis.Dev Biol. 2007 Feb 1;302(1):160-8. doi: 10.1016/j.ydbio.2006.09.016. Epub 2006 Sep 15. Dev Biol. 2007. PMID: 17067567 Free PMC article.
-
RNA binding protein sex-lethal (Sxl) and control of Drosophila sex determination and dosage compensation.Microbiol Mol Biol Rev. 2003 Sep;67(3):343-59, table of contents. doi: 10.1128/MMBR.67.3.343-359.2003. Microbiol Mol Biol Rev. 2003. PMID: 12966139 Free PMC article. Review.
-
Sex determination in Drosophila: The view from the top.Fly (Austin). 2010 Jan-Mar;4(1):60-70. doi: 10.4161/fly.4.1.11277. Epub 2010 Jan 21. Fly (Austin). 2010. PMID: 20160499 Free PMC article. Review.
Cited by
-
Isolation and Characterization of Germline Stem Cells in Protogynous Hermaphroditic Monopterus albus.Int J Mol Sci. 2022 May 24;23(11):5861. doi: 10.3390/ijms23115861. Int J Mol Sci. 2022. PMID: 35682541 Free PMC article.
-
The Dynamic Regulation of mRNA Translation and Ribosome Biogenesis During Germ Cell Development and Reproductive Aging.Front Cell Dev Biol. 2021 Nov 3;9:710186. doi: 10.3389/fcell.2021.710186. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34805139 Free PMC article. Review.
-
Protein Interaction Mapping of Translational Regulators Affecting Expression of the Critical Stem Cell Factor Nos.Dev Reprod. 2017 Dec;21(4):449-456. doi: 10.12717/DR.2017.21.4.449. Epub 2017 Dec 31. Dev Reprod. 2017. PMID: 29354790 Free PMC article.
-
Promiscuity in post-transcriptional control of gene expression: Drosophila sex-lethal and its regulatory partnerships.FEBS Lett. 2017 Jun;591(11):1471-1488. doi: 10.1002/1873-3468.12652. Epub 2017 Apr 28. FEBS Lett. 2017. PMID: 28391641 Free PMC article. Review.
-
Sex, stem cells and tumors in the Drosophila ovary.Fly (Austin). 2013 Jan-Mar;7(1):3-7. doi: 10.4161/fly.22687. Epub 2012 Dec 3. Fly (Austin). 2013. PMID: 23208193 Free PMC article.
References
-
- Chen D, McKearin DM. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003;13:1786–1791. - PubMed
-
- Chen D, McKearin DM. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development. 2003;130:1159–1170. - PubMed
-
- Song X, et al. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–1364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

