Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling
- PMID: 22645348
- PMCID: PMC3390875
- DOI: 10.1073/pnas.1200250109
Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling
Abstract
Human pluripotent stem cells (hPSCs) offer the potential to generate large numbers of functional cardiomyocytes from clonal and patient-specific cell sources. Here we show that temporal modulation of Wnt signaling is both essential and sufficient for efficient cardiac induction in hPSCs under defined, growth factor-free conditions. shRNA knockdown of β-catenin during the initial stage of hPSC differentiation fully blocked cardiomyocyte specification, whereas glycogen synthase kinase 3 inhibition at this point enhanced cardiomyocyte generation. Furthermore, sequential treatment of hPSCs with glycogen synthase kinase 3 inhibitors followed by inducible expression of β-catenin shRNA or chemical inhibitors of Wnt signaling produced a high yield of virtually (up to 98%) pure functional human cardiomyocytes from multiple hPSC lines. The robust ability to generate functional cardiomyocytes under defined, growth factor-free conditions solely by genetic or chemically mediated manipulation of a single developmental pathway should facilitate scalable production of cardiac cells suitable for research and regenerative applications.
Conflict of interest statement
Conflict of interest statement: T.J.K. is a founder and consultant for Cellular Dynamics International, a company that uses human stem cells for drug testing.
Figures


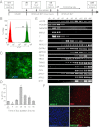

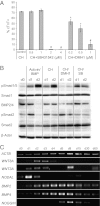


References
-
- Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
-
- Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. - PubMed
-
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Graichen R, et al. Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation. 2008;76:357–370. - PubMed
-
- Yang L, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

