Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire
- PMID: 22645359
- PMCID: PMC3382472
- DOI: 10.1073/pnas.1207934109
Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire
Abstract
Idiosyncratic adverse drug reactions are unpredictable, dose-independent and potentially life threatening; this makes them a major factor contributing to the cost and uncertainty of drug development. Clinical data suggest that many such reactions involve immune mechanisms, and genetic association studies have identified strong linkages between drug hypersensitivity reactions to several drugs and specific HLA alleles. One of the strongest such genetic associations found has been for the antiviral drug abacavir, which causes severe adverse reactions exclusively in patients expressing the HLA molecular variant B*57:01. Abacavir adverse reactions were recently shown to be driven by drug-specific activation of cytokine-producing, cytotoxic CD8(+) T cells that required HLA-B*57:01 molecules for their function; however, the mechanism by which abacavir induces this pathologic T-cell response remains unclear. Here we show that abacavir can bind within the F pocket of the peptide-binding groove of HLA-B*57:01, thereby altering its specificity. This provides an explanation for HLA-linked idiosyncratic adverse drug reactions, namely that drugs can alter the repertoire of self-peptides presented to T cells, thus causing the equivalent of an alloreactive T-cell response. Indeed, we identified specific self-peptides that are presented only in the presence of abacavir and that were recognized by T cells of hypersensitive patients. The assays that we have established can be applied to test additional compounds with suspected HLA-linked hypersensitivities in vitro. Where successful, these assays could speed up the discovery and mechanistic understanding of HLA-linked hypersensitivities, and guide the development of safer drugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


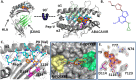
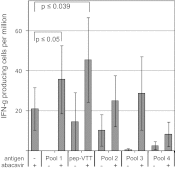
Comment in
-
Explanation for HLA-B*57:01-linked immune-mediated abacavir-induced hypersensitivity.Pharmacogenomics. 2012 Nov;13(14):1567-9. doi: 10.2217/pgs.12.146. Pharmacogenomics. 2012. PMID: 23148633 No abstract available.
References
-
- Rauch A, et al. Prospective genetic screening decreases the incidence of abacavir hypersensitivity reactions in the Western Australian HIV cohort study. Clin Infect Dis. 2006;43:99–102. - PubMed
-
- Chessman D, et al. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity. 2008;28:822–832. - PubMed
-
- Uetrecht J. Idiosyncratic drug reactions: Current understanding. Annu Rev Pharmacol Toxicol. 2007;47:513–539. - PubMed
-
- Phillips EJ, Mallal SA. HLA and drug-induced toxicity. Curr Opin Mol Ther. 2009;11:231–242. - PubMed
-
- Lavergne SN, Park BK, Naisbitt DJ. The roles of drug metabolism in the pathogenesis of T-cell–mediated drug hypersensitivity. Curr Opin Allergy Clin Immunol. 2008;8:299–307. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

